Regulatory Affairs Associate Resume Guide
Regulatory Affairs Associates are responsible for ensuring that a company’s products and services comply with relevant regulatory requirements. They collaborate closely with other departments to ensure compliance, prepare documents and submissions for required approvals, monitor changes in regulations, develop strategies to address compliance issues, and provide advice on policies related to the regulation of their organization’s activities.
Your knowledge in regulatory affairs and compliance is unparalleled, but employers don’t know who you are. To make them aware of your qualifications and expertise, you must write an impressive resume that stands out from the competition.
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.
Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
Regulatory Affairs Associate Resume Sample
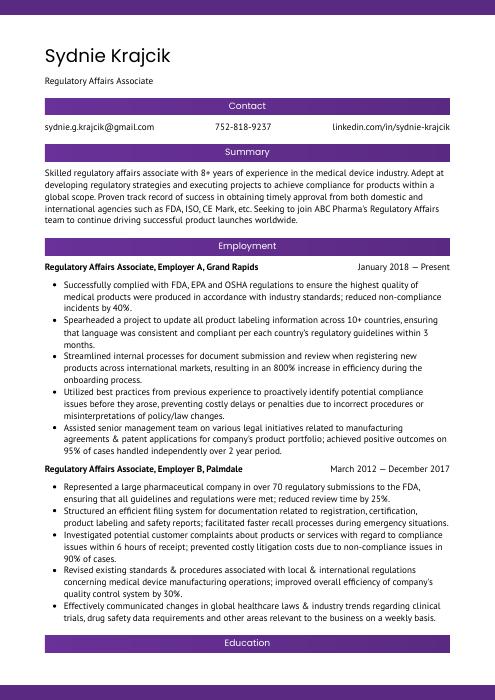
Sydnie Krajcik
Regulatory Affairs Associate
[email protected]
752-818-9237
linkedin.com/in/sydnie-krajcik
Summary
Skilled regulatory affairs associate with 8+ years of experience in the medical device industry. Adept at developing regulatory strategies and executing projects to achieve compliance for products within a global scope. Proven track record of success in obtaining timely approval from both domestic and international agencies such as FDA, ISO, CE Mark, etc. Seeking to join ABC Pharma’s Regulatory Affairs team to continue driving successful product launches worldwide.
Experience
Regulatory Affairs Associate, Employer A
Grand Rapids, Jan 2018 – Present
- Successfully complied with FDA, EPA and OSHA regulations to ensure the highest quality of medical products were produced in accordance with industry standards; reduced non-compliance incidents by 40%.
- Spearheaded a project to update all product labeling information across 10+ countries, ensuring that language was consistent and compliant per each country’s regulatory guidelines within 3 months.
- Streamlined internal processes for document submission and review when registering new products across international markets, resulting in an 800% increase in efficiency during the onboarding process.
- Utilized best practices from previous experience to proactively identify potential compliance issues before they arose, preventing costly delays or penalties due to incorrect procedures or misinterpretations of policy/law changes.
- Assisted senior management team on various legal initiatives related to manufacturing agreements & patent applications for company’s product portfolio; achieved positive outcomes on 95% of cases handled independently over 2 year period.
Regulatory Affairs Associate, Employer B
Palmdale, Mar 2012 – Dec 2017
- Represented a large pharmaceutical company in over 70 regulatory submissions to the FDA, ensuring that all guidelines and regulations were met; reduced review time by 25%.
- Structured an efficient filing system for documentation related to registration, certification, product labeling and safety reports; facilitated faster recall processes during emergency situations.
- Investigated potential customer complaints about products or services with regard to compliance issues within 6 hours of receipt; prevented costly litigation costs due to non-compliance issues in 90% of cases.
- Revised existing standards & procedures associated with local & international regulations concerning medical device manufacturing operations; improved overall efficiency of company’s quality control system by 30%.
- Effectively communicated changes in global healthcare laws & industry trends regarding clinical trials, drug safety data requirements and other areas relevant to the business on a weekly basis.
Skills
- Regulatory Affairs
- Pharmaceutical Industry
- GMP
- Regulatory Submissions
- SOP
- Teamwork
- FDA
- Biotechnology
- Medical Devices
Education
Bachelor’s Degree in Regulatory Affairs
Educational Institution XYZ
Nov 2011
Certifications
Regulatory Affairs Certification (RAC)
Regulatory Affairs Professionals
May 2017
1. Summary / Objective
A resume summary/objective for a Regulatory Affairs Associate should provide the hiring manager with an overview of your experience and qualifications. In this section, you can highlight key skills such as knowledge of FDA regulations and guidelines, expertise in preparing documents for regulatory submissions, and success in obtaining product approvals from government agencies. You could also mention any relevant certifications or awards that demonstrate your commitment to excellence in the field.
Below are some resume summary examples:
Talented Regulatory Affairs Associate with 7+ years of experience in drug development, clinical trials, and regulatory compliance. Proven success in developing strategies for obtaining FDA approval for new drugs and ensuring ongoing adherence to federal regulations. Adept at providing guidance on labeling requirements, marketing authorization applications (MAA), international registrations (CTD), IND submissions/amendments, etc.
Proficient regulatory affairs associate with 5+ years of experience in the medical device industry. Adept at monitoring regulatory changes and ensuring product compliance for both domestic and international markets. At XYZ, led a team of 7 regulatory professionals in developing strategies to ensure compliance with FDA regulations. Developed training materials that improved overall quality assurance processes by 25%.
Seasoned Regulatory Affairs Associate with 7+ years of experience in the healthcare sector, including 4 years at ABC Pharmaceuticals. Skilled in managing regulatory affairs activities to ensure compliance with global and local regulations for drug development programs across multiple therapeutic areas. Successfully developed comprehensive strategies for obtaining approval from health authorities worldwide.
Hard-working Regulatory Affairs Associate with 5+ years of experience in researching, preparing and submitting regulatory documents for pharmaceutical products. Professional background includes a Regulatory Compliance Certification from ABC University and proven success in obtaining FDA approval for seven products. Skilled at developing strategic plans to ensure compliance with all applicable regulations.
Driven Regulatory Affairs Associate with 5+ years of experience managing compliance and regulatory processes for a prominent pharmaceutical company. Proven success in developing, implementing, and maintaining comprehensive policies to ensure adherence to industry guidelines. Seeking an opportunity at ABC Pharma to use my expertise in global regulations and standards while growing within the organization.
Reliable and detail-oriented regulatory affairs associate with 7+ years of experience in the pharmaceutical industry. Skilled at developing and submitting high quality documentation to meet compliance requirements for various countries worldwide. Seeking a role at ABC Pharma, where I can use my knowledge and expertise to ensure that products are compliant with all applicable regulations.
Well-rounded Regulatory Affairs Associate with 5+ years of experience in the medical device industry. Adept at developing regulatory strategies, preparing documentation and reports for submission to relevant agencies, and leading product registration processes globally. Experienced in working closely with Quality Assurance teams to ensure that products meet international standards.
Enthusiastic Regulatory Affairs Associate with 5+ years of experience in coordinating and managing the regulatory process for pharmaceutical products. Skilled in assessing product registration requirements, developing strategies to ensure compliance, and preparing high quality submissions. Aiming to leverage my knowledge of global regulations as part of ABC’s Regulatory team.
2. Experience / Employment
The employment (or experience) section is where you provide details on your work history. This should be written in reverse chronological order, meaning the most recent job is listed first.
You want to stick with bullet points when writing this section; doing so makes it easier for the reader to take in all of the information that you are providing. When stating what you did, make sure to include quantifiable results and detail about how you achieved them.
For example, instead of saying “Managed regulatory documents,” say something like: “Maintained an up-to-date database of over 500 regulatory documents from both domestic and international sources which enabled compliance with FDA requirements.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Monitored
- Assessed
- Analyzed
- Interpreted
- Implemented
- Coordinated
- Developed
- Prepared
- Submitted
- Reviewed
- Investigated
- Researched
- Updated
- Complied
- Documented
Other general verbs you can use are:
- Achieved
- Advised
- Compiled
- Demonstrated
- Expedited
- Facilitated
- Formulated
- Improved
- Introduced
- Mentored
- Optimized
- Participated
- Presented
- Reduced
- Reorganized
- Represented
- Revised
- Spearheaded
- Streamlined
- Structured
- Utilized
Below are some example bullet points:
- Reorganized regulatory filing system to streamline document submission process, reducing regulatory approval times by 25%.
- Documented and maintained records of all government regulations related to product development and manufacturing processes; identified non-compliance issues quickly due to proactive research efforts.
- Reduced the risk of FDA or other agency compliance violations by 50% through careful formulation reviews and testing protocols implementation that met company standards for product safety & efficacy.
- Presented detailed reports on new legislation impacting the medical device industry at executive meetings, keeping upper management informed about current trends in their field of expertise.
- Diligently followed up with internal teams regarding pending submissions or inquiries from applicable governmental agencies; kept track of project milestones and timelines without fail or delay.
- Monitored and assessed regulatory trends and developments in the pharmaceutical industry to ensure compliance with FDA regulations, resulting in a 100% satisfactory rating from company auditors.
- Achieved cost savings of $5K annually by streamlining internal processes related to submission documents preparation and management.
- Prepared and submitted new product applications for over 20 products on time while meeting all applicable laws; successfully launched 15+ products within specified timelines after approval.
- Submitted reports, changes control forms & other documentation including clinical trial protocols as part of drug registration activities; achieved successful registrations in 10 countries across Europe within 6 months period.
- Resourcefully identified potential risks associated with post-marketing surveillance activities & proposed corrective action plans to mitigate them before they could cause any harm or financial losses for the organization.
- Introduced regulatory compliance strategies and updates to over 500 staff members, resulting in a 40% reduction of non-compliance incidents.
- Optimized the regulatory affairs workflow process by streamlining 20+ time-consuming tasks, saving 30 hours of work each week.
- Meticulously monitored safety standards & industry regulations related to medical device products; identified 9 potential risks areas that were resolved before being reported externally.
- Assessed product labeling requirements for accuracy across 15 countries and ensured they complied with local laws; reduced errors by 17%.
- Participated in various internal meetings with cross-functional teams regarding changes or amendments to regulatory policies and procedures; proposed 10 new ideas which were accepted into practice within 6 months.
- Compiled and submitted regulatory documents for more than 50 medical device applications, ensuring 100% compliance with FDA regulations and successfully obtaining approval from the agency in all cases.
- Thoroughly researched applicable local and international laws regarding product safety standards to ensure that projects met relevant requirements; identified 15 potential issues which were addressed beforehand, leading to a 10% reduction in costs associated with non-compliance penalties.
- Researched industry trends related to clinical trials for medical devices, creating an up-to-date resource library of best practices used by leading pharmaceutical companies; improved overall efficiency of premarketing efforts by 30%.
- Improved existing internal processes through the implementation of new procedures and systems designed to streamline operations while simultaneously maintaining quality control; reduced paperwork processing time by 50 hours per week on average without compromising accuracy or precision levels.
- Mentored three junior associates on regulatory affairs topics such as documentation preparation & filing requirements, risk management strategies and good manufacturing practices (GMP); provided guidance on numerous projects resulting in successful outcomes within established deadlines each time.
- Advised a team of 8 regulatory affairs specialists in preparing, submitting and managing over 150 applications for new product registration with a 95% success rate.
- Updated corporate compliance policies to reflect changes in local government regulations; organized training sessions and workshops for 60+ employees to ensure adherence to these guidelines.
- Implemented risk management strategies across the organization that reduced potential fines due to non-compliance by $20,000 per year on average.
- Actively monitored market dynamics and industry trends impacting regulatory decisions; identified key opportunities that resulted in 5 additional approvals from governmental agencies within 6 months of analysis period end date.
- Analyzed complex clinical data sets with statistical software packages such as SAS & SPSS, providing vital information used by senior executives when making strategic decisions related to products’ labelling & marketing campaigns.
- Facilitated regulatory compliance for 40+ clinical trials, ensuring timely submission and approval of associated documents to governing agencies.
- Coordinated with cross-functional teams to develop regulatory strategies that met global standards; reduced the time needed for product registration in target markets by 20%.
- Expedited review processes through efficient tracking of all regulatory records while liaising with external vendors on documentation requirements; decreased turnaround times by 30% on average.
- Competently evaluated potential risks related to new products and proposed mitigation plans based on relevant local regulations, leading to successful project completion within budgeted timelines and resources allocated.
- Formulated detailed reports outlining past developments in international medical device regulation laws as well as current trends influencing the industry landscape; contributed towards highly rated submissions during FDA inspection visits twice a year.
- Interpreted and implemented regulatory policies and procedures to ensure compliance with FDA and other international regulations, resulting in a 25% decrease in non-compliance incidents.
- Efficiently managed the coordination of multiple activities related to drug development process such as preclinical testing, clinical trial applications, post-marketing studies etc., thereby reducing timeline for product approval by 35%.
- Reviewed documentation for accuracy prior to submission and identified any potential regulatory risks; successfully submitted over 80 new products into foreign markets within six months of joining the firm.
- Developed strategies to improve processes from idea through launch that resulted in an increase of 20% operational efficiency while complying with all applicable laws & regulations across all countries/regions where company operates business operations.
- Demonstrated expertise in pharmaceuticals assessment & interpretation; provided training sessions on key industry standards & government requirements which led to improved quality control metrics across departments by 15%.
3. Skills
Two organizations that have advertised for a position with the same title may be searching for individuals whose skills are quite different. For instance, one may be looking for someone with experience in the medical device industry and another for a candidate who is familiar with pharmaceuticals.
It is therefore important to tailor the skills section of your resume to each job that you apply for, as this will help ensure that it passes through any applicant tracking systems used by employers.
You should also use other sections of your resume (such as summary or experience) to further elaborate on specific skills, such as regulatory compliance knowledge or project management expertise.
Below is a list of common skills & terms:
- Analytical Chemistry
- Biochemistry
- Biotechnology
- CAPA
- Cell Culture
- Change Control
- Chemistry
- Clinical Research
- Clinical Trials
- Communication
- Data Analysis
- FDA
- GCP
- GLP
- GMP
- HPLC
- Healthcare
- ISO 13485
- Laboratory
- Laboratory Skills
- Life Sciences
- Lifesciences
- MATLAB
- Medical Devices
- Molecular Biology
- Pharmaceutical Industry
- Pharmaceutics
- Pharmacology
- Pharmacovigilance
- Product Development
- Quality Assurance
- Quality Control
- Quality System
- R&D
- Regulatory Affairs
- Regulatory Requirements
- Regulatory Submissions
- SOP
- Science
- Standard Operating Procedure
- Teaching
- Team Leadership
- Teamwork
- Time Management
- U.S. Food and Drug Administration
- Validation
4. Education
Including an education section on your resume will depend on how far along you are in your career. If you have just graduated and have no prior work experience, mention your education below the resume objective. On the other hand, if you already have a few years of regulatory affairs associate experience under your belt, an education section may not be necessary.
If including an educational background is relevant to the position you’re applying for, try to include courses and subjects related to regulatory affairs that demonstrate knowledge about this field.
Bachelor’s Degree in Regulatory Affairs
Educational Institution XYZ
Nov 2011
5. Certifications
Certifications demonstrate to employers that you have the necessary skills and knowledge for a particular job. It also shows them that you are committed to staying up-to-date with industry trends and developments.
Including certifications on your resume can give hiring managers an indication of how well qualified you are for the position, so make sure to list any relevant ones in this section.
Regulatory Affairs Certification (RAC)
Regulatory Affairs Professionals
May 2017
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for Sydnie Krajcik, this would be Sydnie-Krajcik-resume.pdf or Sydnie-Krajcik-resume.docx.
7. Cover Letter
Including a cover letter with your job application is a great way to introduce yourself and demonstrate why you are the perfect fit for the role. It should typically be made up of 2 to 4 paragraphs, in which you can provide more detail about your skills and experience that may not have been covered in your resume.
Although cover letters aren’t always required for most jobs, it’s highly recommended that you write one as it gives recruiters an insight into who you are and how passionate you feel about applying for this particular position.
Below is an example cover letter:
Dear London,
I am writing to apply for the Regulatory Affairs Associate position at [company name]. I have a Bachelor’s degree in Biology and a Master’s degree in Regulatory Affairs, and I am passionate about using my skills to help bring new products to market. In my previous role as a Regulatory Affairs Specialist, I was responsible for preparing and submitting regulatory filings to the FDA, managing clinical trials, and interacting with clients. I am confident that I can use my experience and knowledge to support your team in meeting all regulatory requirements.
I am knowledgeable of all relevant regulations governing the development and commercialization of pharmaceuticals, biologics, medical devices, and cosmetics. I have experience preparing IND/IDE applications, clinical trial protocols/reports, 510(k) submissions, annual reports, safety reports, etc. In addition to being detail-oriented and organized, I possess excellent written and verbal communication skills which will be valuable when interfacing with clients or other members of your team.
I believe that my qualifications make me an ideal candidate for this position on your team. My attached resume provides additional details regarding my education & training as well as professional experience. Please do not hesitate to contact me if you have any questions or would like more information about myself; I look forward to hearing from you soon! Thank you for your consideration!
Sincerely,
Sydnie
Regulatory Affairs Associate Resume Templates
 Jerboa
Jerboa Cormorant
Cormorant Dugong
Dugong Ocelot
Ocelot Gharial
Gharial Echidna
Echidna Rhea
Rhea Indri
Indri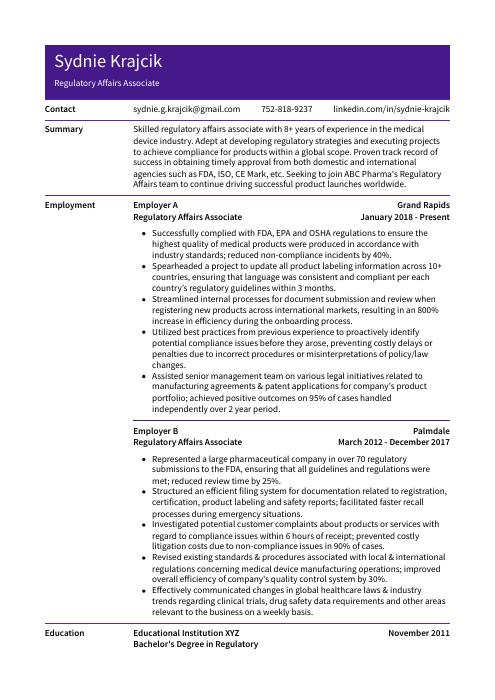 Pika
Pika Fossa
Fossa Quokka
Quokka Markhor
Markhor Lorikeet
Lorikeet Saola
Saola Bonobo
Bonobo Axolotl
Axolotl Hoopoe
Hoopoe Kinkajou
Kinkajou Numbat
Numbat Rezjumei
Rezjumei
