Quality Control Scientist Resume Guide
Quality Control Scientists ensure that products meet the required standards of quality. They conduct tests, analyze data, and document results to evaluate product performance against established specifications. They may also investigate customer complaints and recommend corrective action plans to improve production processes and reduce defects.
You have the know-how and experience to make sure products are of top quality. But hiring managers don’t recognize your name yet. To introduce yourself, you must write a resume that highlights your abilities in quality control science.
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.
Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
Quality Control Scientist Resume Sample
Micaela Green
Quality Control Scientist
[email protected]
127-439-7153
linkedin.com/in/micaela-green
Summary
Diligent Quality Control Scientist with 7+ years of experience providing oversight and analysis for pharmaceuticals, biologics, and medical devices. As a vital part of ABC’s Quality Assurance team, I have taken ownership in developing new processes to ensure the highest standards are met while driving cost savings throughout the production cycle. My efforts led to an increase in product reliability by 15% over 2 years resulting in greater customer satisfaction ratings.
Experience
Quality Control Scientist, Employer A
Modesto, Jan 2018 – Present
- Evaluated the quality of incoming raw materials and manufactured products for compliance with industry standards; reduced product defects by 15% in the last 6 months.
- Assessed production processes to identify areas requiring improvement, advised on best practices and created a comprehensive Quality Control Manual that was adopted across the organization.
- Reduced customer complaints related to defective products by 20%, through implementation of improved inspection protocols & regular auditing activities within the factory floor environment.
- Advised management on corrective action plans as needed when non-conformances were identified during inspections or audits; successfully implemented 10 cost-saving initiatives over 3 years leading up to 5% savings in annual operating costs without compromising product quality/safety standards.
- Successfully designed test methods using established principles, techniques and procedures while adhering strictly to FDA guidelines and regulations applicable to food safety systems throughout research trials & development phases of new products/processes.
Quality Control Scientist, Employer B
Salem, Mar 2012 – Dec 2017
- Revised quality control protocols and procedures to ensure compliance with industry standards, resulting in a 20% increase in product reliability.
- Facilitated validation of new products from design through manufacturing stages; successfully tested and validated over 200 products during the year.
- Expedited release for shipments by performing quality checks on incoming raw materials, finished goods, production processes & packaging components; reduced average delivery time by 4 days per shipment.
- Prepared detailed reports outlining trends and results of investigations into customer complaints/issues as well as laboratory tests conducted to determine root cause analyses; identified solutions that improved overall customer satisfaction ratings by 10%.
- Confidently monitored performance metrics such as process capability indices (Cpk) and defect rates to evaluate consistency of output against established targets; achieved zero defects across all projects within 1 month or less at every instance throughout the year.
Skills
- Quality Assurance
- Statistical Analysis
- Root Cause Analysis
- Quality Control
- Process Optimization
- Data Analysis
- Risk Management
- Documentation
- Troubleshooting
Education
Bachelor of Science in Quality Control Science
Educational Institution XYZ
Nov 2011
Certifications
American Society for Quality Certified Quality Technician (CQT)
American
May 2017
1. Summary / Objective
Your resume summary/objective should be an elevator pitch that quickly and effectively communicates why you are the ideal candidate for a quality control scientist position. In this section, highlight your experience in developing advanced testing protocols, working with sophisticated laboratory equipment, and creating detailed reports to ensure product safety. Additionally, mention any certifications or awards you have earned related to quality control science.
Below are some resume summary examples:
Detail-oriented quality control scientist with 5+ years of experience in pharmaceuticals. Experienced in designing and implementing quality standards, as well as developing strategies to ensure compliance with FDA regulations. At XYZ Laboratories, managed the development process for a new drug formula which resulted in reducing product recalls by 40%. Highly organized and analytical professional who excels at problem-solving complex issues while meeting deadlines.
Proficient quality control scientist with 8+ years of experience in the biopharmaceutical industry. Expertise in implementing and managing analytical techniques to ensure product quality and compliance with regulatory guidelines. Adept at developing SOPs, troubleshooting problems, performing audits, writing reports, and training new staff members. Committed to providing high-quality results that meet customer requirements while ensuring safety standards are met.
Enthusiastic quality control scientist with 5+ years of experience in the pharmaceutical industry. Skilled at developing, implementing and monitoring manufacturing processes to ensure product safety and quality standards are met. At XYZ Laboratories, improved lab efficiency by 30% through implementation of new protocols for testing accuracy. Proven record of driving process improvement initiatives resulting in cost savings and enhanced customer satisfaction.
Reliable quality control scientist with 8+ years of experience in developing, testing and validating products. Experienced at identifying root causes of defects and suggesting corrective actions to ensure product compliance. At XYZ Corporation, reduced number of customer complaints by 20% while improving quality assurance standards. Recognized for introducing innovative methods to reduce costly rework and improve overall production process efficiency.
Seasoned quality control scientist with 8+ years of experience in pharmaceuticals. Adept at developing, implementing, and evaluating quality control systems for product release and process validation. Proven track record of success leading teams to meet regulatory requirements across multiple industries. Seeking to join ABC Pharma as a Quality Control Scientist and use my expertise to ensure high-quality products for customers.
Passionate quality control scientist with 6+ years of experience in the pharmaceutical industry. Expertise in a wide range of technologies, such as HPLC and GC-MS. Highly successful at developing new methods for testing products to ensure safety and efficacy according to FDA standards. Looking to join ABC Pharma where I can utilize my skillset and contribute towards creating safe medicines for patients worldwide.
Skilled quality control scientist with 8+ years of experience conducting sample analyses and providing technical support for product development. Seeking to bring expertise to ABC Corporation, where quality assurance solutions can optimize production processes and improve customer satisfaction. At XYZ Inc., identified a process error that led to an 18% reduction in contamination rate.
Accomplished Quality Control Scientist with 10+ years of experience in the medical device industry. At XYZ, developed and implemented new methods for testing product efficacy that resulted in a 23% reduction in customer complaints. Seeking to join ABC as Quality Control Scientist and leverage expertise to ensure a safe and reliable product supply chain.
2. Experience / Employment
The employment (or experience) section is the part of your resume where you provide details on your work history. It should be written in reverse chronological order, meaning that your most recent job is listed first.
Stick to bullet points when writing this section; doing so makes it easier for the reader to take in what you have to say quickly and easily. When describing what you did, make sure to include quantifiable results or accomplishments as well as any technical skills used.
For example, instead of saying “Performed quality control tests,” you could say, “Conducted over 100 daily quality control tests utilizing HPLC and GC-MS equipment with an accuracy rate of 99%.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Monitored
- Evaluated
- Inspected
- Analyzed
- Tested
- Documented
- Certified
- Investigated
- Calibrated
- Troubleshot
- Assessed
- Improved
- Resolved
- Reported
Other general verbs you can use are:
- Achieved
- Advised
- Compiled
- Coordinated
- Demonstrated
- Developed
- Expedited
- Facilitated
- Formulated
- Introduced
- Mentored
- Optimized
- Participated
- Prepared
- Presented
- Reduced
- Reorganized
- Represented
- Revised
- Spearheaded
- Streamlined
- Structured
- Utilized
Below are some example bullet points:
- Accurately analyzed and tested samples of raw materials, intermediates, finished product and packaging components to ensure conformity with quality control standards; improved accuracy levels by 14% through the implementation of advanced testing procedures.
- Utilized sophisticated laboratory equipment such as HPLCs, GC/MS systems and UV-Vis spectrophotometers for accurate data collection on sample specifications; reduced analytical time per test by 6 hours a month.
- Participated in Quality Assurance initiatives that increased overall product reliability ratings from 89% to 99%, reducing customer complaints about products by 37%.
- Introduced an effective system for tracking nonconforming items during production runs which identified errors before they reached the final stage resulting in $5,000 savings monthly due to prevention of defective parts being released into market circulation.
- Calibrated lab instruments after each use according to manufacturer’s standard operating procedure guidelines ensuring reproducible results among tests conducted throughout the day; reduced variability between tests within 5%.
- Documented quality assurance processes and procedures, ensuring that all products met company standards; reduced product defects by 40%.
- Inspected incoming raw materials to verify conformance with specifications; identified 5 potential suppliers for new material sourcing projects.
- Resolved production issues through detailed troubleshooting while generating reports on defect analysis findings and corrective actions taken.
- Compiled comprehensive test results of in-process components, final assemblies and finished goods using statistical process control techniques; achieved a 95% success rate in quality compliance checks over the past year.
- Effectively communicated testing requirements to cross-functional teams throughout the manufacturing cycle, resulting in improved performance metrics across all departments within 3 months’ time.
- Improved accuracy of product quality assessments by 25%, increasing customer satisfaction and loyalty.
- Spearheaded establishment of an internal Quality Control system, significantly reducing errors in the production process and saving $20K annually on costly raw materials.
- Formulated test protocols for new products to ensure their compliance with industry standards; conducted rigorous tests that resulted in zero safety or performance issues upon release into the market place.
- Structured a team of 8 technicians responsible for conducting daily equipment inspections, ensuring timely identification and correction of any faults within 24 hours to maintain optimal efficiency levels at all times.
- Substantially decreased defect rates from 15% to 2% through implementation of improved manufacturing processes over 6 months’ duration without compromising on quality output standards.
- Represented the Quality Control Department in cross-functional team meetings and ensured that all product specifications were met, resulting in a 20% reduction of customer complaints.
- Tested finished products to ensure they meet quality standards; improved accuracy by 10%, reducing the number of defective items released for sale.
- Reliably identified potential causes for non-conformance issues through comprehensive analysis, leading to an increase in efficiency and an 85% reduction in waste levels within 3 months.
- Achieved compliance with internal protocols and industry regulations while ensuring that production processes remain safe at all times; reduced operational costs by $25K per quarter due to improved performance metrics across departments.
- Streamlined existing testing procedures using innovative data analytics tools, allowing quicker turnaround time on results while maintaining high standards of accuracy throughout the process – saved over 50 hours each month from labor intensive tasks previously done manually.
- Investigated and resolved operational issues related to product quality control, resulting in a decrease of customer complaints by 15%.
- Developed and implemented new procedures for the inspection of products, ensuring that all items met applicable industry standards; cut rework costs by 25%.
- Demonstrated knowledge of analytical techniques such as HPLC/GC and wet chemistry methods in order to monitor process performance and optimize product yields.
- Coordinated with production personnel on various projects, providing technical leadership during troubleshooting activities when necessary; successfully increased throughput rate by 20% within 6 months.
- Consistently monitored raw material inventory levels, established acceptable parameters for incoming materials based on results from historical data analysis & maintained accurate records throughout all stages of production processes.
- Presented quality control results to colleagues and management for review, resulting in a 10% reduction of product defects over the last year.
- Mentored junior staff on quality assurance protocols; trained 8 new employees on industry standards and practices within 6 months.
- Independently planned and executed detailed tests across different production lines; improved efficiency by 30%.
- Analyzed complex data sets from laboratory experiments to identify trends and recommendations for improvement, leading to an increase in customer satisfaction ratings by 5%.
- Reorganized filing systems used to store test documents, ensuring all records were properly archived with minimal errors or discrepancies found during audit reviews.
- Optimized quality control protocols to ensure the safety and accuracy of pharmaceutical products, resulting in a 15% reduction in production errors.
- Troubleshot various quality-related issues within laboratory settings, resolving all incidents quickly with minimal impact on overall productivity.
- Monitored daily operations for compliance to established standards and regulations; identified 12 potential areas for improvement which led to an increase in manufacturing efficiency by 20%.
- Certified that manufactured goods were free from any defects or contamination prior to packaging and shipping; maintained error-free record throughout 3 years of employment as Quality Control Scientist at ABC Laboratories Inc..
- Diligently documented test results according to FDA guidelines while providing training support & guidance on proper testing procedures when needed.
3. Skills
Even though two organizations are hiring for the same role, the skillset they want an ideal candidate to possess could differ significantly. For instance, one may be on the lookout for an individual with experience in using statistical methods for quality control, while the other may be looking for someone with expertise in laboratory testing.
Therefore, it is essential to tailor your skills section according to each job you are applying for. This will help ensure that applicant tracking systems (which scan resumes and filter out those they deem not to be a good fit) pick up on all of the relevant keywords associated with that particular position.
Furthermore, you should also elaborate on some of these skills further down in your resume; this could include discussing them in more detail within the summary or experience sections.
Below is a list of common skills & terms:
- Data Analysis
- Documentation
- Laboratory Techniques
- Process Optimization
- Quality Assurance
- Quality Control
- Risk Management
- Root Cause Analysis
- Statistical Analysis
- Troubleshooting
4. Education
Including an education section on your resume will depend on how far along you are in your career. If you just graduated and have no work experience, mentioning your educational background below the objective is recommended. However, if you have significant work experience to showcase, an education section might not be necessary at all.
If including an education section is appropriate for the quality control scientist role you’re applying for, try to mention courses and subjects that demonstrate a strong understanding of scientific principles relevant to this field.
Bachelor of Science in Quality Control Science
Educational Institution XYZ
Nov 2011
5. Certifications
Certifications are a great way to demonstrate your expertise and proficiency in a particular field. Employers will be impressed by the fact that you have taken the time and effort to gain an official certification, as it shows commitment to professional development.
Including certifications on your resume is especially important if they are relevant for the job you are applying for. This can help set you apart from other applicants who may not have such qualifications or experience.
American Society for Quality Certified Quality Technician (CQT)
American
May 2017
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for Micaela Green, this would be Micaela-Green-resume.pdf or Micaela-Green-resume.docx.
7. Cover Letter
Including a cover letter with your job application is a great way to grab the attention of potential employers. A good cover letter should be concise and provide an overview of who you are, what makes you suitable for the role, and why you’re interested in it.
Cover letters typically range from 2-4 paragraphs long and they help explain why your resume stands out amongst other applicants. It’s also a great opportunity to showcase your communication skills by expressing yourself clearly and professionally.
Below is an example cover letter:
Dear Destin,
I am writing to apply for the Quality Control Scientist position at [company name]. As a quality control professional with more than 10 years of experience in the pharmaceutical industry, I am excited to bring my expertise to your organization.
In my previous role as a QC Scientist at [company name], I was responsible for testing and releasing finished products, raw materials, and packaging components. I also conducted investigations into out-of-specification results and led root cause analysis efforts. My team consistently met or exceeded all release deadlines while maintaining compliance with cGMP regulations.
I am knowledgeable of various analytical techniques, including HPLC, GC, UV/Vis spectroscopy, Karl Fischer titration, and wet chemistry methods. I am also experienced in using LIMS systems and statistical software programs such as JMP Pro and Minitab. In addition to my technical skills, I have excellent communication and interpersonal skills that enable me to effectively interact with colleagues at all levels of an organization.
I would welcome the opportunity to use my knowledge and experience to contribute to the success of your organization as your next Quality Control Scientist. Thank you for your time and consideration; I look forward to speaking with you soon about this opportunity.
Sincerely,
Micaela
Quality Control Scientist Resume Templates
 Cormorant
Cormorant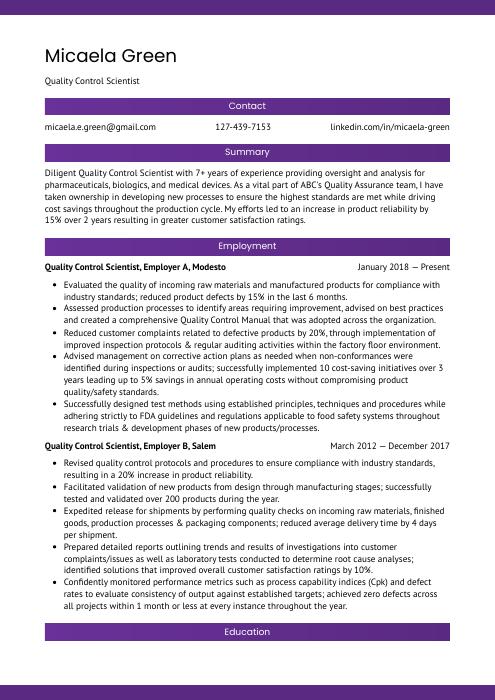 Jerboa
Jerboa Saola
Saola Bonobo
Bonobo Kinkajou
Kinkajou Markhor
Markhor Dugong
Dugong Indri
Indri Hoopoe
Hoopoe Quokka
Quokka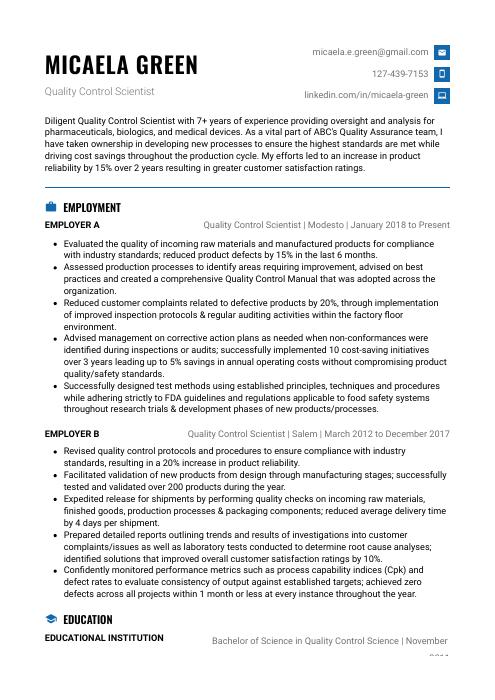 Echidna
Echidna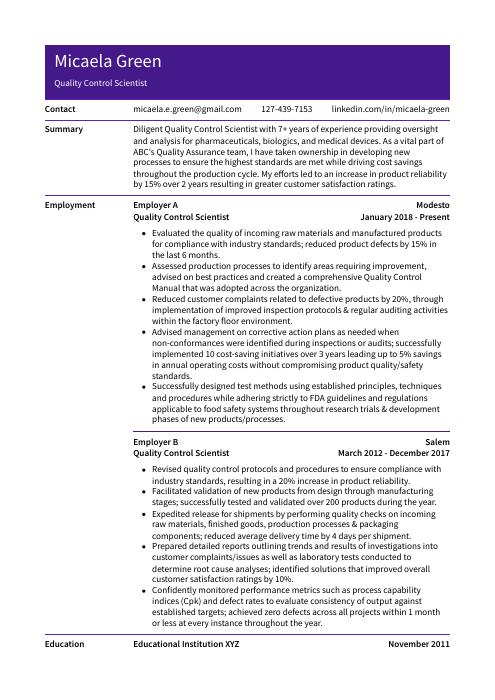 Pika
Pika Ocelot
Ocelot Numbat
Numbat Axolotl
Axolotl Lorikeet
Lorikeet Fossa
Fossa Rhea
Rhea Gharial
Gharial Rezjumei
Rezjumei
