Quality Control Chemist Resume Guide
Quality control chemists ensure the quality of products by performing tests and inspections. They analyze raw materials, in-process samples, and finished goods to verify that they meet specifications. They also perform calibration checks on instruments used for testing and troubleshoot any problems with product or equipment.
You have an impressive eye for detail and a knack for finding discrepancies in samples. But employers don’t know who you are yet, so to make them aware of your talents, you must craft a resume that stands out from the competition.
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.
Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
Quality Control Chemist Resume Sample
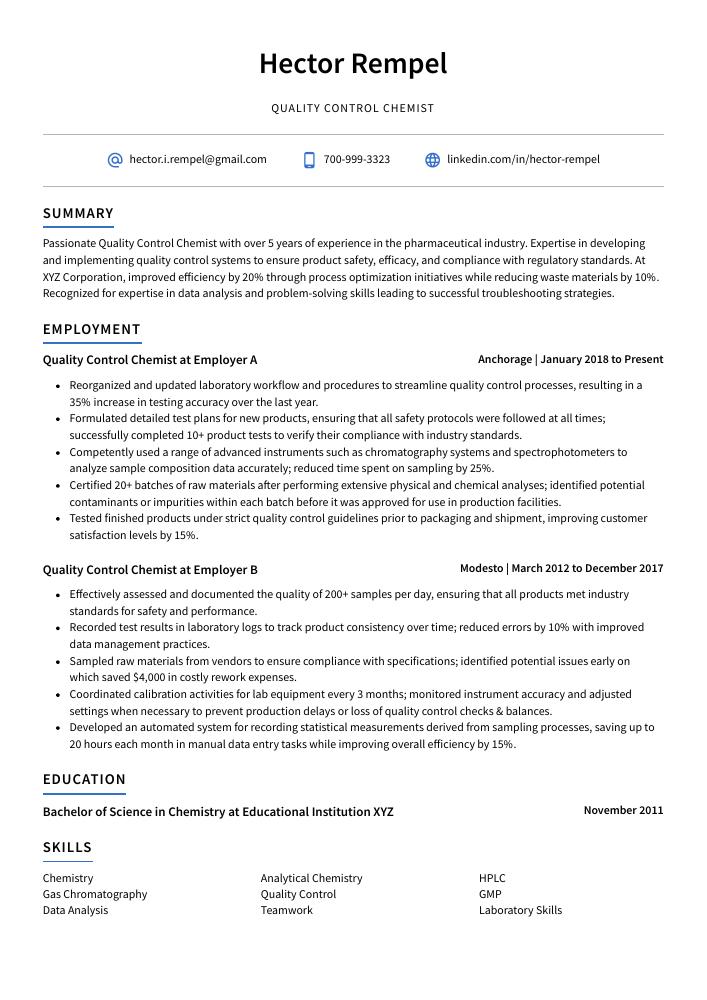
Hector Rempel
Quality Control Chemist
[email protected]
700-999-3323
linkedin.com/in/hector-rempel
Summary
Passionate Quality Control Chemist with over 5 years of experience in the pharmaceutical industry. Expertise in developing and implementing quality control systems to ensure product safety, efficacy, and compliance with regulatory standards. At XYZ Corporation, improved efficiency by 20% through process optimization initiatives while reducing waste materials by 10%. Recognized for expertise in data analysis and problem-solving skills leading to successful troubleshooting strategies.
Experience
Quality Control Chemist, Employer A
Anchorage, Jan 2018 – Present
- Reorganized and updated laboratory workflow and procedures to streamline quality control processes, resulting in a 35% increase in testing accuracy over the last year.
- Formulated detailed test plans for new products, ensuring that all safety protocols were followed at all times; successfully completed 10+ product tests to verify their compliance with industry standards.
- Competently used a range of advanced instruments such as chromatography systems and spectrophotometers to analyze sample composition data accurately; reduced time spent on sampling by 25%.
- Certified 20+ batches of raw materials after performing extensive physical and chemical analyses; identified potential contaminants or impurities within each batch before it was approved for use in production facilities.
- Tested finished products under strict quality control guidelines prior to packaging and shipment, improving customer satisfaction levels by 15%.
Quality Control Chemist, Employer B
Modesto, Mar 2012 – Dec 2017
- Effectively assessed and documented the quality of 200+ samples per day, ensuring that all products met industry standards for safety and performance.
- Recorded test results in laboratory logs to track product consistency over time; reduced errors by 10% with improved data management practices.
- Sampled raw materials from vendors to ensure compliance with specifications; identified potential issues early on which saved $4,000 in costly rework expenses.
- Coordinated calibration activities for lab equipment every 3 months; monitored instrument accuracy and adjusted settings when necessary to prevent production delays or loss of quality control checks & balances.
- Developed an automated system for recording statistical measurements derived from sampling processes, saving up to 20 hours each month in manual data entry tasks while improving overall efficiency by 15%.
Skills
- Chemistry
- Analytical Chemistry
- HPLC
- Gas Chromatography
- Quality Control
- GMP
- Data Analysis
- Teamwork
- Laboratory Skills
Education
Bachelor of Science in Chemistry
Educational Institution XYZ
Nov 2011
Certifications
Certified Quality Control Chemist (CQCC)
American Society
May 2017
1. Summary / Objective
A resume summary/objective is a great way to give the hiring manager an overview of your qualifications as a quality control chemist. In this section, you can highlight your experience in laboratory testing and analysis, any certifications or awards you have received for excellence in quality assurance, and how you successfully identified issues with production processes that resulted in cost savings.
Below are some resume summary examples:
Seasoned Quality Control Chemist with extensive experience in developing and implementing testing procedures for pharmaceuticals. Skilled in ensuring product safety, efficacy, and compliance to cGMP standards. At XYZ Pharmaceuticals identified a labeling discrepancy that reduced production costs by an estimated $6,000/month. Seeking to leverage analytical mindset at ABC Labs where I can help develop innovative solutions while maintaining the highest quality of products possible.
Reliable quality control chemist with 8+ years of experience in laboratory testing and quality assurance. Experienced working with a variety of chemicals, raw materials and finished products to ensure safety standards are met. At XYZ Company, conducted over 1000 tests on incoming raw materials for contamination levels and identified issues before they reached production stages. Familiar with industry regulation requirements for product manufacturing processes and protocols.
Detail-oriented quality control chemist with 5+ years of experience performing analytical and physical tests in the pharmaceutical field. Experienced in developing Quality Control systems, tracking trends, and creating reports to ensure high product quality standards are met. At XYZ Corporation, successfully identified a potential contamination issue that saved $45K in recall costs. Committed to upholding industry regulations for product safety and efficacy.
Professional quality control chemist with 10 years of experience in product development and quality assurance. Experienced in performing various types of testing to ensure the safety, efficacy, and stability of products. Proven track record for developing innovative solutions to reduce costs while improving overall quality standards. Highly organized with excellent problem-solving skills; able to work independently or as part of a team.
Accomplished quality control chemist with 8+ years of experience conducting analytical testing and inspections for medical device companies. Seeking to join ABC Medical Lab to develop quality assurance protocols in accordance with FDA regulations, while also utilizing chemical analysis methods such as HPLC and GC. In prior roles, achieved 97% on-time completion rate for all assigned tasks and improved product reliability by 10%.
Determined and driven quality control chemist with 5+ years of experience in the biopharmaceutical industry. Proven track record in developing and executing laboratory testing protocols to ensure product safety, purity, efficacy, and compliance with regulatory guidelines. Experienced in using advanced analytical techniques such as HPLC/GCMS/UPLC-MS/UV spectroscopy for analysis of raw materials and finished products.
Diligent quality control chemist with 5+ years of experience in the pharmaceutical industry. Proficient at designing and executing experiments, analyzing data, interpreting results and providing recommendations for improvement to ensure compliance with quality standards. Currently seeking a position at ABC Pharma where I can apply my knowledge of chemical processes to bring value to the organization.
Driven quality control chemist with 7+ years of experience in the chemical industry. Skilled at setting up and conducting experiments, analyzing data, interpreting results, troubleshooting problems, and developing new methods for testing products. Looking to join ABC Industries where I can utilize my expertise to ensure product quality is maintained according to regulatory standards.
2. Experience / Employment
The work history/experience section should be written in reverse chronological order, with your most recent job listed first. Stick to bullet points when talking about what you did; this makes it easier for the reader to take in the information quickly and easily.
When writing out each of these bullet points, provide detail on what you did and any quantifiable results that were obtained from your efforts. For example, instead of saying “Analyzed samples,” say something like “Performed chemical analyses on 20+ samples per day using HPLC/GC-MS techniques resulting in a 99% accuracy rate.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Analyzed
- Inspected
- Monitored
- Tested
- Evaluated
- Certified
- Documented
- Calibrated
- Investigated
- Sampled
- Recorded
- Assessed
- Adjusted
- Reported
Other general verbs you can use are:
- Achieved
- Advised
- Compiled
- Coordinated
- Demonstrated
- Developed
- Expedited
- Facilitated
- Formulated
- Improved
- Introduced
- Mentored
- Optimized
- Participated
- Prepared
- Presented
- Reduced
- Reorganized
- Represented
- Revised
- Spearheaded
- Streamlined
- Structured
- Utilized
Below are some example bullet points:
- Demonstrated superior attention to detail when performing quality control tests on raw materials, chemicals, manufactured products and environmental samples; achieved a 99.7% accuracy rate in test results.
- Achieved significant savings by ensuring that production processes are compliant with all applicable health & safety regulations; conducted over 250 compliance audits within the last year with zero violations reported.
- Calibrated laboratory instruments and maintained testing equipment according to manufacturer specifications; trained 3 new team members in proper maintenance procedures for glassware, pH meters & titrators over 4 weeks.
- Thoroughly evaluated final products against established standards and inspected incoming goods from suppliers against contractual requirements; identified 6 defective products which were returned back or reworked before delivery to customers, saving $1K+ in losses due to customer complaints/returns of substandard goods each month.
- Inspected production premises regularly for cleanliness and hazardous material issues as part of risk assessment process; implemented corrective actions where necessary which reduced potential contamination risks by 70%.
- Prepared and analyzed quality control tests for different chemicals and products to ensure that they met the required industry standards, resulting in a 20% reduction of defective items.
- Presented monthly reports on test results to senior management team; identified areas of improvement and proposed cost-effective solutions.
- Represented the company at professional events such as conferences and seminars by presenting cutting-edge research in the field of quality testing & assurance techniques; gained positive feedback from 200+ attendees over two consecutive years.
- Actively monitored production line processes (including temperature, pressure, humidity etc.) using specialized equipment; ensured compliance with safety regulations whilst improving product durability by 10%.
- Participated in developing new protocols for incoming materials inspection process based on customer satisfaction survey responses which increased repeat orders by 5%.
- Compiled and maintained detailed records of over 500 quality control tests and inspections, ensuring compliance with industry regulations.
- Confidently identified potential contaminants in raw materials and products through careful analysis; prevented an estimated 15% product defects from being released to the market.
- Revised existing testing protocols for accuracy and efficiency, implementing changes that saved up to $4,000 per quarter on laboratory supplies costs.
- Introduced a new set of proactive sample collection techniques across all production lines which reduced the rate of contamination by 13%.
- Mentored 4 junior chemists on proper safety procedures when conducting quality control experiments; successfully completed 20+ certification courses together.
- Monitored and adjusted quality control processes to ensure compliance with GMP regulations and industry standards, resulting in a 15% decrease in laboratory errors.
- Independently tested incoming raw materials for contamination and analyzed finished products using advanced analytical instruments; identified impurities that exceeded safety thresholds on over 50 occasions.
- Investigated customer complaints regarding defective products, leading to the successful resolution of 10 product disputes within 1 week each time.
- Analyzed lab results from all departments across the production line to guarantee consistent quality throughout manufacturing operations; maintained an accuracy rate of 98%.
- Utilized HPLC/UPLC equipment to detect minute differences between batches of drugs at various stages of development, achieving significant cost savings by identifying process flaws early on (+$10K).
- Optimized testing procedures and methods in the laboratory to ensure accurate results, resulting in a 10% reduction of testing time per sample.
- Evaluated product quality by conducting experiments with various chemicals, recording data and interpreting test results; identified potential risks or defects which enabled preventative measures to be taken.
- Reliably ran experiments according to safety protocols while working independently as well as collaboratively within a team environment; conducted over 1,000 tests for contaminants each month without any misreadings or errors.
- Structured comprehensive reports detailing findings from research projects and presented them effectively at regular meetings with senior management members & other stakeholders; improved project completion rate by 25%.
- Facilitated training sessions on topics related to chemical analysis techniques such as gas chromatography-mass spectrometry (GC-MS) for new employees entering the Quality Control department; decreased onboarding costs by $500 annually due to reduced need for outside consultants/trainers.
- Utilized analytical chemistry techniques, including chromatography and spectroscopy, to evaluate the physical properties of raw materials and finished products in order to ensure adherence to quality standards; identified over 40 defective items within a span of one month.
- Reduced re-testing turnaround time by 50% through implementation of advanced automation processes for data analysis across various production lines.
- Expedited review process by devising an efficient system for reporting laboratory observations accurately and consistently; improved overall lab productivity by 15%.
- Diligently conducted routine inspections on all incoming shipments from suppliers according to established protocols, thereby mitigating risk associated with product contamination or mislabeling issues before they reach final customers.
- Developed innovative testing methods that are more reliable than existing approaches used in current industry practices; reduced error rate from 14% down to 3%.
- Advised and guided production staff on quality control best practices, resulting in a 10% increase of product compliance with industry regulations.
- Improved the accuracy of laboratory testing and analysis operations by introducing new protocols; reduced error rate from 9% to 4%.
- Documented all test findings and investigated customer complaints regarding product quality; identified defects that led to a 20% improvement in overall product performance within 3 months.
- Spearheaded an initiative to streamline the labeling process for incoming raw materials, reducing inspection wait times by 25%.
- Resourcefully utilized existing lab equipment to perform detailed tests on finished goods without additional investments, saving over $15,000 annually in costs related to purchasing specialized tools and consumables supplies.
3. Skills
Two organizations that have advertised for a position with the same title may be searching for individuals whose skills are quite different. For instance, one may be looking for someone with experience in laboratory analysis and another for someone who is proficient with statistical methods.
It’s essential to tailor the skills section of your resume to each job you are applying for, as many employers use applicant tracking systems these days. The purpose of such programs is to scan resumes for certain keywords before passing them on to a human being.
In addition, it’s important that you elaborate on the most relevant skills in other areas of your resume – such as the summary or experience sections – so that recruiters can get an accurate picture of what kind of quality control chemist you are and how well suited you would be for their organization.
Below is a list of common skills & terms:
- Analytical Chemistry
- Biochemistry
- Biology
- Biotechnology
- Cell Culture
- Chemistry
- Chromatography
- Communication
- Data Analysis
- English
- FDA
- FTIR
- GC MS
- GLP
- GMP
- Gas Chromatography
- Good Laboratory Practice
- HPLC
- High Performance Liquid Chromatography
- IR Spectroscopy
- LIMS
- Laboratory
- Laboratory Skills
- Mass Spectrometry
- Microbiology
- Molecular Biology
- NMR
- Organic Chemistry
- PCR
- Pharmaceutical Industry
- Quality Assurance
- Quality Control
- R&D
- Raw Materials
- SOP
- Science
- Spectroscopy
- Standard Operating Procedure
- Teaching
- Team Leadership
- Teamwork
- Time Management
- Titration
- UV/Vis
- UV/Vis Spectroscopy
- Validation
- Wet Chemistry
4. Education
Including an education section on your resume will depend on how far along you are in your career. If you just graduated and have no work experience, mention your education below the resume objective. However, if you have plenty of work experience to showcase, it might be better to omit the education section altogether.
If an education section is included, try to list courses and subjects that are related to quality control chemistry as well as any relevant certifications or qualifications earned while studying for this role.
Bachelor of Science in Chemistry
Educational Institution XYZ
Nov 2011
5. Certifications
Certifications are a great way to demonstrate your expertise in a particular field. They show potential employers that you have gone the extra mile and taken the time to prove yourself as an expert in your chosen profession.
Including certifications on your resume is especially important if they are relevant to the job you’re applying for, or if it demonstrates specialized knowledge that could give you an edge over other applicants.
Certified Quality Control Chemist (CQCC)
American Society
May 2017
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for Hector Rempel, this would be Hector-Rempel-resume.pdf or Hector-Rempel-resume.docx.
7. Cover Letter
Providing a cover letter to accompany your resume is a great way to showcase yourself during the job application process. It helps you stand out from other candidates and gives recruiters more insight into who you are and why you’re the right person for the role.
A cover letter should include 2 to 4 paragraphs that provide more detail than what’s already mentioned in your resume. For example, it can be used as an opportunity to explain any gaps in employment history or clarify something about your qualifications or experience which could otherwise be misinterpreted if left unaddressed.
Below is an example cover letter:
Dear Jan,
I am writing to apply for the Quality Control Chemist position at [company name]. As a chemist with 7+ years of experience conducting quality control tests, I am confident I will make valuable contributions to your organization.
In my previous role as a QC chemist at [company name], I was responsible for conducting physical and chemical tests on raw materials, in-process products, and finished products. I also developed and validated new test methods, as well as maintained existing methods. My work helped ensure that products met all relevant specifications and standards.
Through my work, I have gained extensive knowledge of cGMPs and SOPs. In addition, I have developed strong attention to detail and excellent problem-solving skills. These skills would enable me to effectively contribute to the quality control function at your company.
I look forward to discussing how my experience can benefit your organization in more detail. Thank you for your time and consideration; I look forward to hearing from you soon.
Sincerely,
Hector
Quality Control Chemist Resume Templates
 Jerboa
Jerboa Markhor
Markhor Lorikeet
Lorikeet Indri
Indri Fossa
Fossa Ocelot
Ocelot Rhea
Rhea Hoopoe
Hoopoe Kinkajou
Kinkajou Pika
Pika Saola
Saola Quokka
Quokka Numbat
Numbat Dugong
Dugong Cormorant
Cormorant Axolotl
Axolotl Echidna
Echidna Bonobo
Bonobo Gharial
Gharial Rezjumei
Rezjumei