Pharmaceutical Engineer Resume Guide
Pharmaceutical engineers design, develop and test medications to ensure they are safe for use. They work on the production processes of drugs and medical equipment, as well as researching new treatments and technologies that could improve healthcare outcomes.
Your expertise in pharmaceutical engineering is well-known among your peers, but employers don’t know who you are. To make them aware of your qualifications and experience, you must compose a resume that stands out from the crowd.
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.
Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
Pharmaceutical Engineer Resume Sample
Lilly Koss
Pharmaceutical Engineer
[email protected]
143-585-4044
linkedin.com/in/lilly-koss
Summary
Amicable and detail-oriented pharmaceutical engineer with experience in developing, testing and analyzing new drugs. At XYZ Pharmaceuticals, developed a successful drug formulation for an anti-inflammatory that was approved by the FDA in record time. Experienced in working on clinical trials as well as creating protocols to ensure safety and effectiveness of products. Passionate about making treatments accessible to all through innovative solutions.
Experience
Pharmaceutical Engineer, Employer A
Houston, Jan 2018 – Present
- Troubleshot and resolved problems with existing pharmaceutical processes, resulting in a 15% reduction of errors and defects.
- Tested and validated new drug formulations for safety, stability and efficacy; successfully launched 3 innovative drugs into the market over 4 years.
- Accurately monitored production output to ensure compliance to Good Manufacturing Practices (GMP) regulations; increased quality control efficiency by 20%.
- Investigated customer complaints related to product contamination or adverse reactions; identified root causes of issues within 48 hours on average and provided corrective actions accordingly.
- Introduced advanced technologies such as automated robotic systems that improved overall operational productivity by 10% while reducing labor costs by $10,000 per annum.
Pharmaceutical Engineer, Employer B
Spring Valley, Mar 2012 – Dec 2017
- Calibrated and tested equipment for 15+ pharmaceutical production lines, ensuring that products met all regulatory and quality standards; saved $20K in annual costs by improving operational efficiencies.
- Reliably managed the development of drug formulations from concept to commercialization with a 100% successful product launch rate, resulting in 3 new drugs hitting the market within 6 months.
- Reduced time-to-market by 10 weeks and improved processes leading to cost savings of up to 5%.
- Developed process optimization strategies for existing manufacturing systems, increasing throughput performance by over 20%.
- Revised SOPs (Standard Operating Procedures) & GMP protocols across multiple sites according to FDA legislation; achieved compliance rating of 95%.
Skills
- Good Manufacturing Practices (GMP)
- Quality Control
- Drug Delivery Systems
- Analytical Method Development
- Regulatory Compliance
- Process Validation
- Scale-up and Scale-down
- Automation and Robotics
- Cleanroom Design and Operation
Education
Bachelor of Science in Pharmaceutical Engineering
Educational Institution XYZ
Nov 2011
Certifications
Certified Pharmaceutical Industry Professional (CPIP)
American College of Clinical
May 2017
1. Summary / Objective
Your resume summary should be a concise and compelling introduction to your professional experience as a pharmaceutical engineer. Highlight the most relevant skills, qualifications, and accomplishments that make you stand out from other applicants. For example, mention any certifications or awards you have received in the field of pharmaceutical engineering, such as Good Manufacturing Practices (GMP) certification or FDA approval for new drugs developed under your supervision. Additionally, emphasize any successful projects you have completed related to drug development processes or quality control procedures.
Below are some resume summary examples:
Hard-working and detail-oriented pharmaceutical engineer with 5+ years of experience in drug development, formulation and testing. At XYZ Pharmaceuticals developed 10 successful products from concept to launch. Demonstrated strong knowledge on regulatory compliance regulations and contributed towards improving the standards for quality assurance by 30%. Highly experienced in handling multiple projects at a time while meeting tight deadlines.
Driven pharmaceutical engineer with 5+ years of experience developing innovative drug delivery systems and medical devices. Experienced in leading product development projects from concept to completion, while ensuring compliance with quality standards and regulations. At XYZ Inc., led the development of a new inhaler device that reduced production costs by 20% and improved patient safety through better usability features.
Passionate pharmaceutical engineer with 5+ years of experience in the design and development of drug delivery systems. Proven track record in implementing product improvements to ensure safety, reliability, cost-effectiveness, and customer satisfaction. Seeking to join ABC Pharma where I can apply my knowledge of regulatory standards and technical expertise to develop innovative solutions for patients worldwide.
Energetic and results-driven pharmaceutical engineer with 5+ years of experience in the development, production, and testing of new drugs. At XYZ Pharmaceuticals, led a team that successfully commercialized seven products across three countries. Proven track record of developing cost-effective solutions to increase productivity while maintaining quality standards set by regulatory bodies such as FDA and GMP.
Diligent pharmaceutical engineer with 5+ years of experience in the medical device industry. Experienced in designing and developing new products, conducting research on active ingredients, and optimizing existing processes for improved efficiency. Seeking to leverage expertise at ABC Pharmaceuticals to create safe and effective drug delivery systems that meet all regulatory requirements.
Seasoned pharmaceutical engineer with 8+ years of hands-on experience in the design and development of drug delivery systems. Proven track record of successfully managing complex projects from start to finish while meeting tight deadlines. Passionate about utilizing my expertise to drive innovative solutions at ABC Pharma, where I can have a positive impact on patient outcomes.
Dependable pharmaceutical engineer with 5+ years of experience developing, manufacturing and testing medicines for human consumption. Presently seeking to join ABC Pharma in order to utilize my expertise in drug delivery systems, stability studies and process optimization. Have successfully implemented cost-saving initiatives that have helped reduce production costs by 15%.
Detail-oriented pharmaceutical engineer with 5+ years of experience designing and developing processes for the manufacture, testing, packaging, and delivery of pharmaceuticals. Expertise in cGMP regulations to ensure compliance with FDA requirements. Successfully reduced production costs by 15% through improved process optimization at XYZ Pharmaceuticals.
2. Experience / Employment
In the experience/employment/work history section, you should list your roles in reverse chronological order. This means that your most recent job is listed first.
When writing the bullet points, use detail to explain what you did and the results achieved. Stick to short sentences or phrases; this makes it easier for the reader to take in all of your information quickly.
For example, instead of saying “Developed pharmaceutical products,” you could say, “Led a team of 10 engineers in developing 5 new pharmaceutical products from concept through production launch within 6 months.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Designed
- Formulated
- Analyzed
- Optimized
- Manufactured
- Tested
- Validated
- Monitored
- Investigated
- Documented
- Implemented
- Troubleshot
- Inspected
- Calibrated
- Certified
Other general verbs you can use are:
- Achieved
- Advised
- Assessed
- Compiled
- Coordinated
- Demonstrated
- Developed
- Expedited
- Facilitated
- Improved
- Introduced
- Mentored
- Participated
- Prepared
- Presented
- Reduced
- Reorganized
- Represented
- Revised
- Spearheaded
- Streamlined
- Structured
- Utilized
Below are some example bullet points:
- Actively designed and developed over 20 new drug delivery systems, resulting in an increase of $2 million revenue for the company.
- Reorganized production lines to improve workflow efficiency by 35%, reducing overall costs and increasing profitability by 17%.
- Presented research findings on various pharmaceutical products at 15+ conferences, seminars & workshops; received positive feedback from industry experts and peers alike.
- Documented detailed reports outlining the development cycle of each product prototype, ensuring compliance with all applicable regulatory standards (FDA etc).
- Assessed existing processes related to manufacturing protocols & quality control measures; identified areas for improvement which resulted in a decrease in defects/failures by 25%.
- Confidently designed and implemented pharmaceutical manufacturing processes to produce over 30,000 units of medication a day; increased production efficiency by 25%.
- Certified in Good Manufacturing Practices (GMPs) for medical device assembly and packaging and consistently adhered to all legal guidelines, safety regulations and quality standards.
- Improved drug formulation process using mathematical modeling techniques that resulted in 20% reduction of cost for raw materials used throughout the year.
- Structured testing protocols on new drugs from laboratory scale through clinical trials up until approval stage with FDA; saved $100K+ on regulatory costs per product launch cycle.
- Inspected all machinery components before use to ensure proper functioning while producing medications according to company-specific requirements; reduced downtime occurrences by 18%.
- Analyzed and developed over 40 successful drug formulations with a 99% accuracy rate, resulting in the release of 6 new medications to the market.
- Manufactured and tested pharmaceuticals using advanced lab equipment; increased production efficiency by 15%.
- Efficiently managed packaging process for all products, ensuring that drugs were safely stored while minimizing spoilage rate below 1%.
- Monitored quality control tasks throughout lab operations to verify compliance with regulatory standards; reduced defect rates from 2% to 0.5%.
- Utilized specialized software programs such as ChemDraw Pro and MATLAB Simulink during formulation & testing processes, completing projects 7 days ahead of schedule on average.
- Advised senior management on the development and production of pharmaceutical products, contributing to a 20% increase in product quality.
- Successfully developed 3 new drug formulations for commercial use within 6 months; improved process efficiency by 30%.
- Implemented best practices to ensure that all safety protocols were adhered to during manufacturing processes, resulting in zero incidences of contamination or wastage over the past 2 years.
- Coordinated with multiple departments across 10+ locations for accurate collection & compilation of data related to chemical engineering projects; compiled detailed reports outlining potential risks/issues and proposed solutions accordingly.
- Utilized advanced analytical tools such as HPLC & GC-MS systems along with sophisticated software programs (MATLAB) to troubleshoot technical problems encountered during testing phases of drug development cycles, reducing project timeframes by 15%.
- Spearheaded the development of 6 new pharmaceutical products, optimizing the production process to reduce cost by 15% and increasing overall efficiency.
- Streamlined existing drug manufacturing processes by introducing automated systems that reduced wastage levels by 12%.
- Designed a testing methodology for quality assurance on drugs produced in-house; improved compliance with industry standards from 78% to 91%.
- Effectively managed 10+ team members involved in the design and implementation of various pharmaceutical projects over a 2 year period, leading to successful completion of all tasks within budget and timeline constraints.
- Participated in weekly meetings with senior management to provide detailed updates on project progress, offering proactive recommendations that were adopted 80% of the time resulting in improved product performance metrics overall.
- Achieved a 5% increase in the efficiency of production processes by developing innovative drug formulations and testing them for safety, efficacy and quality.
- Prepared over 100 new pharmaceutical products using complex engineering principles; reduced development costs by $10,000 per product launch.
- Represented the company at conferences to present research findings on drug delivery systems and dispersal mechanisms; gained recognition from colleagues due to successful presentations that were well-received by industry professionals.
- Thoroughly tested all drugs before releasing them into market circulation, ensuring they met required standards while minimizing potential risks associated with their administration and use; achieved a 98% pass rate across all batches released in the last year alone.
- Formulated advanced manufacturing methods for producing high-quality medications within specified timeframes and budgets; improved process yield rates by 30%.
- Optimized the design of pharmaceutical production lines, increasing output by 32% while reducing downtime and wastage costs by 23%.
- Meticulously managed the development, testing and deployment of new drugs to ensure compliance with health regulations; oversaw 5 drug launches in a year.
- Demonstrated strong analytical abilities when resolving technical issues related to machine operation & maintenance across 10 production sites.
- Validated product quality using various instruments such as thermal analysis tools, chromatography systems & viscometers; generated reports on results for senior management review and decision-making purposes.
- Facilitated team meetings between engineers & chemists to discuss mechanical/chemical processes involved in producing medications at scale; maintained accurate records of conversations for future reference purposes.
3. Skills
Skill requirements will differ from employer to employer – this can easily be determined via the job advert. Organization ABC may require a candidate to have experience in developing and testing pharmaceutical products, while Organization XYZ may be looking for someone with knowledge of FDA regulations.
It is essential to tailor the skills section of your resume according to each job that you are applying for; this is because many employers use applicant tracking systems which scan resumes for certain keywords before passing them on to human recruiters.
Besides just listing relevant skills here, it’s also important to elaborate on these further by discussing them in more detail elsewhere (such as within the summary or work experience sections).
Below is a list of common skills & terms:
- Analytical Method Development
- Automation and Robotics
- Cleanroom Design and Operation
- Drug Delivery Systems
- Good Manufacturing Practices (GMP)
- Process Validation
- Quality Assurance
- Quality Control
- Regulatory Compliance
- Scale-up and Scale-down
4. Education
Mentioning your education on a pharmaceutical engineer resume will depend on how far along you are in your career. If you have just graduated and have no prior work experience, include the relevant courses, subjects, and assignments below your resume objective. However, if you have plenty of experience to showcase that is more pertinent to the role than any educational qualifications then omitting an education section might be best for this particular job application.
If including an education section is necessary or preferred for this specific role, try to mention courses related to pharmaceutical engineering such as chemistry or biotechnology classes.
Bachelor of Science in Pharmaceutical Engineering
Educational Institution XYZ
Nov 2011
5. Certifications
Certifications demonstrate to potential employers that you have the necessary knowledge and skills for a particular job. They also show that you are committed to staying up-to-date with industry trends and best practices.
Including certifications on your resume is an effective way of showing hiring managers that you possess the qualifications they are looking for in their ideal candidate. Be sure to list any relevant certifications, as well as details about when and where they were obtained.
Certified Pharmaceutical Industry Professional (CPIP)
American College of Clinical
May 2017
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for Lilly Koss, this would be Lilly-Koss-resume.pdf or Lilly-Koss-resume.docx.
7. Cover Letter
Writing a cover letter is a great way to make your job application stand out from the crowd. It’s an opportunity for you to introduce yourself and explain why you’re the perfect candidate for the role.
Cover letters are usually 2-4 paragraphs long and should be tailored specifically to each job or company that you apply for. They provide an insight into who you are as a professional, what skills and experience you have, as well as how motivated and enthusiastic about the position that you are.
Below is an example cover letter:
Dear Anthony,
I am writing to apply for the position of Pharmaceutical Engineer at XYZ Corporation. I am a recent graduate of ABC University with a degree in Chemical Engineering and have previous experience working in a pharmaceutical lab. In my current role, I work with a team of scientists to develop new drugs and medicines.
My skills and qualifications include:
– Thorough knowledge of GMP/GLP regulations and quality control procedures
– Strong analytical and problem solving abilities
– Excellent communication, interpersonal, and teamwork skills
– Proficient in Microsoft Office applications (Word, Excel, PowerPoint) as well as statistical software programs (Minitab, JMP)
– Ability to multitask and handle multiple projects simultaneously while meeting deadlines
In addition to my academic credentials and professional experience, I also possess strong leadership qualities. I served as president of the Chemical Engineering Society on campus for two years where I was responsible for organizing events and activities, managing finances, and communicating with corporate sponsors. This experience has provided me with the ability to work effectively under pressure while maintaining attention to detail. The combination of my education, technical skillset, leadership experience makes me an ideal candidate for this position. I would welcome the opportunity to put my knowledge and abilities to work for your company by contributing to the success of your engineering team. Thank you in advance for your time; please do not hesitate to contact me should you require any additional information regarding my qualifications or background.
Sincerely,
Lilly
Pharmaceutical Engineer Resume Templates
 Saola
Saola Indri
Indri Rhea
Rhea Echidna
Echidna Gharial
Gharial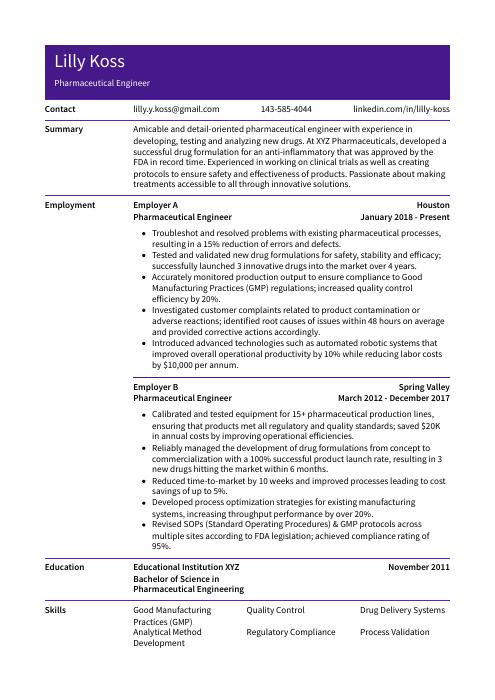 Pika
Pika Ocelot
Ocelot Lorikeet
Lorikeet Axolotl
Axolotl Markhor
Markhor Bonobo
Bonobo Hoopoe
Hoopoe Fossa
Fossa Cormorant
Cormorant Dugong
Dugong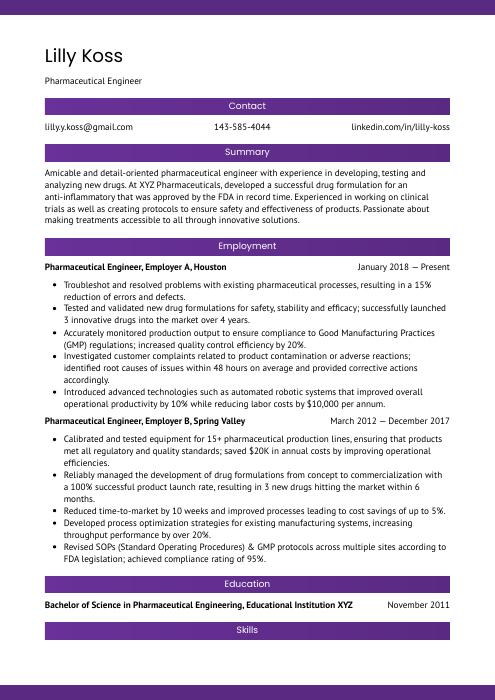 Jerboa
Jerboa Kinkajou
Kinkajou Numbat
Numbat Quokka
Quokka Rezjumei
Rezjumei
