Bioprocess Technician Resume Guide
Bioprocess Technicians work in a laboratory setting to monitor, operate and maintain bioprocess systems. They are responsible for the production of pharmaceuticals, vaccines and other products derived from living organisms or their components. They also perform testing on these products as well as troubleshoot any issues that may arise during production.
You’re a master of bioprocessing and have the experience to prove it. But hiring managers don’t know who you are, so crafting an impressive resume is essential for getting yourself noticed. Showcase your expertise in this field by writing a standout resume today!
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.
Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
Bioprocess Technician Resume Sample
Brannon Thiel
Bioprocess Technician
[email protected]
209-366-8201
linkedin.com/in/brannon-thiel
Summary
Diligent Bioprocess Technician with 10+ years of experience in developing and maintaining biopharmaceutical production processes. Skilled at troubleshooting, validating, and controlling highly complex equipment used for large-scale manufacturing operations. At XYZ, reduced downtime by 25% through the implementation of preventive maintenance protocols. Awarded Employee of the Year for exceptional performance in 2018 & 2019.
Experience
Bioprocess Technician, Employer A
Fullerton, Jan 2018 – Present
- Analyzed and monitored bioprocesses and equipment performance to ensure manufacturing operations ran smoothly; reduced downtime by 15%.
- Accurately measured, weighed and prepared reagents according to strict GMP guidelines; increased productivity of the process by 25%.
- Formulated solutions for various laboratory experiments involving cell culture, fermentation processes and downstream separations; improved efficiency of quality control procedures by 10%.
- Adjusted parameters such as temperature, pressure & pH levels within established limits for optimal product output with minimal wastage; saved an average $5K per month in production costs.
- Inspected all processing instruments regularly for proper functioning prior to use; decreased safety risks associated with faulty equipment usage by 30% over 3 months’ period.
Bioprocess Technician, Employer B
Fremont, Mar 2012 – Dec 2017
- Advised bioprocess engineers on the most effective methods to cultivate and maintain cell cultures, resulting in a 25% increase in productivity.
- Reduced bacterial contamination by 50%, through developing and implementing an optimized maintenance schedule for laboratory equipment and bioreactors.
- Disassembled bioreactor systems, performed preventive maintenance tasks such as replacing pumps, valves and other components; reduced down time by 20%.
- Competently operated lab instruments including pH meters, spectrophotometers & HPLC’s to conduct routine analyses of samples; documented results accurately with no errors or discrepancies after 5 years of experience working as a Bioprocess Technician.
- Revised Standard Operating Procedures (SOPs) related to the operation of lab equipment & biological processes; contributed toward reducing production costs by $10K over 6 months’ period.
Skills
- Cell Culture
- Fermentation
- Chromatography
- Analytical Techniques
- Quality Control
- Data Analysis
- Good Manufacturing Practices
- Process Optimization
- Troubleshooting
Education
Bachelor of Science in Bioprocess Technology
Educational Institution XYZ
Nov 2011
Certifications
Certified Bioprocess Technician
International Society for Pharmaceutical Engineering (
May 2017
1. Summary / Objective
A resume summary for a bioprocess technician should be an effective pitch to the hiring manager. It should provide them with a snapshot of your experience and qualifications, such as your ability to operate complex laboratory equipment, troubleshoot technical issues in production processes, and adhere to safety protocols. You can also mention any certifications or awards you have achieved that demonstrate your commitment to excellence in this field.
Below are some resume summary examples:
Accomplished bioprocess technician with 10 years of experience in the biotechnology industry. Adept at troubleshooting and maintaining equipment, developing operational procedures, and optimizing processes to improve efficiency. Proven ability to work collaboratively while ensuring quality control standards are upheld. Seeking a position as a Bioprocess Technician at XYZ Company where I can utilize my skillset for success.
Hard-working bioprocess technician with 5 years of experience in manufacturing and research & development. Experienced in operating large-scale bioreactors, troubleshooting process issues, and performing sample analysis for quality control. At XYZ Corporation, led a team to develop new protocols that improved production efficiency by 15%. Expertise includes protein purification/isolation and cell culture maintenance.
Enthusiastic bioprocess technician with 5+ years of experience in biotechnology. Skilled at executing lab protocols, maintaining equipment and supplies, and conducting research to improve processes. At ABC Inc., developed a new method for culturing cells that increased yields by 30%. Experienced in microbial techniques such as fermentation, cell culture, chromatography purification, enzyme assays and PCR analysis.
Amicable bioprocess technician with 6+ years of experience in biotechnology and pharmaceuticals. Skilled in operating, troubleshooting, repairing, and maintaining lab equipment used for chemical processes. Experienced with a wide range of analytical instruments such as HPLCs and GCMS systems. Successfully increased production efficiency by 20% at XYZ Pharmaceuticals through process optimization initiatives.
Proficient Bioprocess Technician with 7+ years of experience in biopharmaceutical manufacturing. Skilled in aseptic technique, cell culture processing and downstream unit operations. Seeking to join ABC Biotech as the next Senior Process Technician where I can utilize my technical skills and knowledge to help improve production efficiency while ensuring safety compliance standards are met at all times.
Professional bioprocess technician with 5 years of experience in the food and beverage industry. Skilled at troubleshooting, calibrating and maintaining bioreactors, centrifuges and other related equipment. Proven ability to improve process efficiency by 15% through optimizing fermentation tanks configurations. Committed to providing timely production reports for management decision-making processes.
Talented bioprocess technician with 5+ years of industrial experience in biotechnological production. Proficient in cell culture, fermentation, purification and sterilization processes; developed new techniques to improve process efficiency by 15%. Seeking a challenging role at ABC Biotech to implement innovative solutions for their wide range of products.
Energetic and experienced bioprocess technician with 10+ years of experience working in a variety of production environments and utilizing various laboratory techniques. Skilled at troubleshooting and optimizing processes to ensure efficient operations. Seeking to apply expertise towards the success of ABC BioTech as an integral part of their team.
2. Experience / Employment
In the experience section, you should list your employment history in reverse chronological order. This means that your most recent job is listed first.
For this section, it’s best to stick with bullet points for each role; doing so makes the information easier to digest quickly. When writing these bullets, think about what you did and any quantifiable results achieved as a result of your work.
For example, instead of saying “Performed bioprocessing experiments,” you could say “Conducted over 100 bioprocessing experiments involving cell culture growth optimization and protein purification processes which resulted in an average yield increase of 40%.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Monitored
- Operated
- Calibrated
- Troubleshot
- Analyzed
- Assembled
- Inspected
- Processed
- Sterilized
- Sampled
- Recorded
- Adjusted
- Tested
- Prepared
- Disassembled
Other general verbs you can use are:
- Achieved
- Advised
- Assessed
- Compiled
- Coordinated
- Demonstrated
- Developed
- Expedited
- Facilitated
- Formulated
- Improved
- Introduced
- Mentored
- Optimized
- Participated
- Presented
- Reduced
- Reorganized
- Represented
- Revised
- Spearheaded
- Streamlined
- Structured
- Utilized
Below are some example bullet points:
- Sterilized and maintained cleanroom environment according to cGMP and other regulatory requirements, ensuring the safety of bioprocess equipment and reducing cross-contamination risks by 40%.
- Structured processes for cell culture development, fermentation optimization, downstream purification & process validation; increased efficiency rate in laboratory operations by 30% without compromising quality control standards.
- Reliably operated a wide range of sophisticated lab instruments such as spectrophotometers, flow cytometers and chromatographs to analyze samples accurately; decreased error rates from 5% to 0.5%.
- Improved protein production yields significantly through continuous monitoring and troubleshooting during manufacturing runs; saved up to $10K in raw material costs annually due to optimized operating procedures.
- Introduced experimental techniques such as high throughput screening (HTS) into daily laboratory workflows which resulted in 50+ new research projects completed within 6 months at a fractional cost compared with traditional methods used previously.
- Recorded, monitored, and adjusted bioprocess parameters such as pH, temperature, oxygen concentration and flow rate to ensure optimal operation of the system; reduced production time by 10% on average.
- Assembled and operated laboratory equipment including centrifuges, autoclaves, incubators and bioreactors in compliance with safety regulations; completed over 200 assembly tasks within two months without any incidents or errors.
- Presented regular reports on process performance metrics to management teams using data analysis software tools like SPSS for informed decision-making purposes; improved accuracy of report findings by 15%.
- Successfully conducted experiments related to cell culture media optimization & product purification processes resulting in an increase of protein yields from 5 grams/liter to 8 grams/liter in a month’s time frame.
- Expedited biotherapeutics manufacturing operations through automated techniques such as robotic dispensing systems while ensuring strict adherence to quality control standards set forth by regulatory bodies; achieved 95% efficiency rating at every audit inspection cycle over the last year.
- Tested multiple bioprocess operations like fermentation, purification and cell-culture to ensure compliance with safety regulations and quality standards; improved product yield by 17% in the last quarter.
- Coordinated closely with scientists and technicians to monitor process parameters such as pH level, temperature, nutrient levels etc., while troubleshooting any issues or equipment malfunctions that arose during production.
- Assessed new technologies for potential implementation within the existing bioprocessing system and proposed innovative solutions for improving operational efficiency of plant facilities; reduced downtime by 10 hours per month on average.
- Efficiently managed laboratory inventory including chemicals & reagents required for daily sample testing activities while ensuring proper storage conditions were maintained at all times; decreased costs associated with disposal of expired products by $5,000 annually.
- Represented organization at technical conferences/seminars related to biotechnology advancements in order to stay abreast of latest industry trends & techniques; increased overall knowledge base regarding modern bio-processing methods used across multiple industries.
- Spearheaded process optimization initiatives that reduced bioprocessing times by 25% and improved overall productivity of the laboratory.
- Optimized workflows for critical tests, resulting in a 20% reduction in process errors and cost savings of $10K per month.
- Streamlined testing protocols based on feedback from other technicians to improve quality control standards; achieved 99+% accuracy rate with each batch tested.
- Diligently monitored cell culture & fermentation processes, ensuring all batches met stringent safety specifications within allotted time frames; decreased contamination incidents by 75%.
- Developed detailed documentation tracking system to monitor progress of experiments/samples throughout entire production cycle, increasing visibility into operations performance across team members.
- Demonstrated strong technical and problem solving capabilities when operating laboratory equipment, troubleshooting bioprocessing experiments and monitoring process parameters; reduced processing time by 25% on average.
- Sampled bioreactor cultures at various stages of the production cycle to measure product concentration levels, testing for purity in accordance with established protocols and standards.
- Participated actively in design reviews for new manufacturing processes, providing valuable feedback that led to a 20% improvement in yield rate over two months period of implementation.
- Actively maintained cleanroom environment according to GMP guidelines while performing cell culture operations; improved operational efficiency by 15%.
- Monitored fermentation conditions closely using chromatography techniques & other analytical instruments; identified potential problems quickly resulting in 30+ successful batches produced within the last year alone.
- Achieved 99.9% accuracy in chemical reactions and bioprocessing operations by calibrating laboratory equipment such as pumps, centrifuges, incubators and pH meters.
- Operated fermentation systems to grow microorganisms at optimal temperatures and monitored cell growth during the production process; decreased product spoilage rate by 20%.
- Effectively managed a team of 6 technicians while handling complex tasks such as setting up assay parameters and troubleshooting instrument malfunctions; completed projects 2 weeks ahead of schedule on average.
- Utilized analytical instruments like liquid chromatographs, spectrophotometers & microscopes to analyze samples for quality control purposes and achieved 100% accuracy each time when assessing results against standards set forth by regulatory agencies.
- Ensured accurate data collection from various experiments conducted using statistical tools such as SPSS software packages; reduced errors in predictions by 8%.
- Compiled and analyzed over 200 different types of data from bioprocess experiments, providing key insights to project managers and supervisors that resulted in a 10% increase in operational efficiency.
- Facilitated the development and optimization of new fermentation processes for various microorganisms; produced high yield cultures with successful cell growth rates on five separate occasions.
- Thoroughly documented all laboratory procedures related to bioreactor operations such as sterilization, inoculation, sample collection & analysis, pH control and nutrient/feedstock addition for future reference.
- Mentored junior lab technicians on proper safety protocols when working with bio-hazardous materials; improved quality assurance scores by 17%.
- Prepared detailed reports summarizing experimental results obtained through PCR testing utilizing chromatography techniques; reduced time spent analyzing samples by 25%.
- Reorganized bioprocess operations by re-designing equipment layouts, resulting in a 15% decrease in cycle time for production.
- Processed and monitored up to 500 liters of bioreactor solutions per day according to company protocols; ensured that all batches adhered to safety standards with zero contamination incidents reported over the past 6 months.
- Troubleshot process issues quickly and accurately using industry-standard tools such as pH meters, spectrophotometers and chromatographs; successfully identified root causes of problems 75% faster than average technicians within the department.
- Meticulously logged data into laboratory notebooks during experiments, providing valuable insights that contributed towards increasing efficiency by 12%.
3. Skills
Even though two organizations are hiring for the same role, the skillset they want an ideal candidate to possess could differ significantly. For instance, one may be on the lookout for an individual with experience in bioprocessing and another may be looking for someone with knowledge of chemical engineering.
Your skills section should reflect this by listing the most relevant qualifications that you possess, as well as any other related ones. This way, employers will have a clear understanding of what makes you an ideal candidate for their position.
It is also important to remember that many companies use applicant tracking systems these days; therefore, it is essential to include keywords from the job posting so your resume can pass through them successfully and reach human eyes! Additionally, make sure to elaborate on some key skills in other sections such as the summary or experience section.
Below is a list of common skills & terms:
- Analytical Techniques
- Cell Culture
- Chromatography
- Data Analysis
- Equipment Maintenance
- Fermentation
- Good Manufacturing Practices
- Process Optimization
- Quality Control
- Troubleshooting
4. Education
Adding an education section on your resume will depend on how far along you are in your career. If you just graduated and have no prior experience, mention your education below or near the top of your resume objective. However, if you already have a few years of work experience under your belt, an education section might not be necessary at all.
If including an education section is appropriate for the job application, try to list courses related to bioprocess technology that demonstrate skills relevant to the position.
Bachelor of Science in Bioprocess Technology
Educational Institution XYZ
Nov 2011
5. Certifications
Certifications are a great way to demonstrate your knowledge and proficiency in a certain field. They are especially important for jobs that require specific skills or qualifications.
Including certifications on your resume can help you stand out from other applicants, as it shows potential employers that you have taken the time and effort to become certified in an area relevant to the job they are hiring for. Make sure to list any certifications related directly to the position so they know exactly what skill set you bring with you.
Certified Bioprocess Technician
International Society for Pharmaceutical Engineering (
May 2017
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for Brannon Thiel, this would be Brannon-Thiel-resume.pdf or Brannon-Thiel-resume.docx.
7. Cover Letter
Cover letters are a great way to make yourself stand out from the competition when applying for jobs. They are typically 2 to 4 paragraphs in length and serve as an introduction that explains why you’re interested in the role, what skills and experience you possess, and how your qualifications can benefit the company.
Whilst cover letters are not always mandatory for job applications, they provide employers with more information about who you are beyond your resume. This allows them to get a better understanding of your personality, values and ambitions before making their final decision on which candidate is best suited for the job.
Below is an example cover letter:
Dear Trevor,
I am writing to apply for the position of Bioprocess Technician at Genentech. With a degree in Biology and experience working in a cGMP environment, I am confident that I would excel in this role.
In my previous role as a Research Associate at XYZ Company, I was responsible for conducting experiments and analyzing data related to the development of new drugs. My experience with cell culture, HPLC, and other laboratory techniques will be valuable in this role. In addition, my excellent organizational skills and attention to detail will ensure that all bioprocesses are carried out correctly and efficiently.
I am excited about the opportunity to use my skills and knowledge in a more applied setting. I am confident that I can make a positive contribution to your team and look forward to contributing to the success of Genentech. Thank you for your time and consideration; please do not hesitate to contact me if you have any questions or need any additional information.
Sincerely,
Brannon
Bioprocess Technician Resume Templates
 Fossa
Fossa Rhea
Rhea Kinkajou
Kinkajou Saola
Saola Ocelot
Ocelot Dugong
Dugong Indri
Indri Numbat
Numbat Pika
Pika Jerboa
Jerboa Quokka
Quokka Lorikeet
Lorikeet Bonobo
Bonobo Axolotl
Axolotl Gharial
Gharial Echidna
Echidna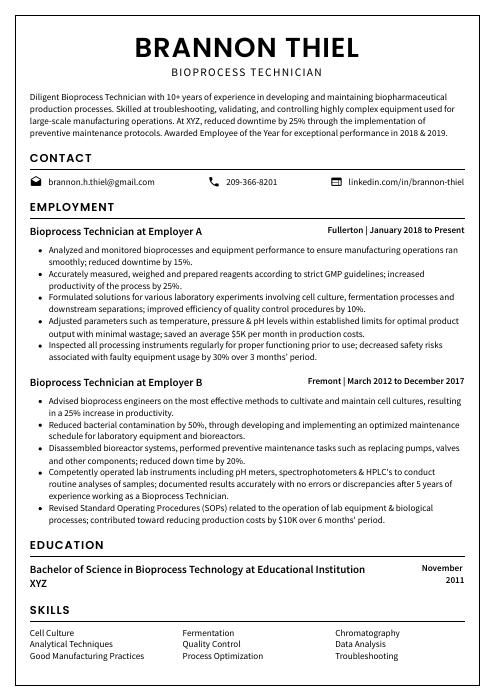 Cormorant
Cormorant Markhor
Markhor Hoopoe
Hoopoe Rezjumei
Rezjumei
