Biomedical Engineer Resume Guide
Biomedical engineers design and develop medical equipment, such as artificial organs, prostheses, instruments and software used in the diagnosis and treatment of diseases. They analyze biological problems to determine how best to use engineering principles for healthcare purposes. Additionally, they work with doctors and other professionals to create solutions that improve patient care outcomes.
It’s no secret that you have a knack for biomedical engineering. To get your foot in the door of this competitive industry, you must write a resume that emphasizes all your qualifications and experience.
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.
Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
Biomedical Engineer Resume Sample
General Mertz
Biomedical Engineer
[email protected]
101-961-2591
linkedin.com/in/general-mertz
Summary
Hard-working biomedical engineer with 5+ years of experience in the medical device industry. Skilled problem-solver, capable of developing innovative solutions to complex problems. At XYZ, led a team that designed and developed a new cardiac catheterization system which resulted in an increase of 10% efficiency compared to existing technology. Received accolades from peers for my research on improving patient safety measures using biomedical engineering tools and techniques.
Experience
Biomedical Engineer, Employer A
Tallahassee, Jan 2018 – Present
- Proficiently designed, developed and tested medical devices such as surgical implants, prosthetics, pacemakers and dialysis machines; reduced time-to-market for new products by 10%.
- Structured clinical trials to evaluate the safety and efficacy of a wide range of biomedical equipment; increased acceptance rate from regulatory authorities by 15%.
- Demonstrated expertise in developing cost effective solutions while ensuring patient safety requirements were met or exceeded at all times.
- Facilitated cross functional teams composed of technicians, scientists & engineers to improve current product designs; saved $50K in production costs over 2 years through process optimization initiatives.
- Reduced troubleshooting time for complex medical systems from 6 hours down to 4 hours on average using advanced diagnostic tools & software programs designed specifically for diagnostics within healthcare environments.
Biomedical Engineer, Employer B
Bridgeport, Mar 2012 – Dec 2017
- Coordinated multiple medical device projects for a team of 10 biomedical engineers, resulting in the successful launch of 3 products with an average cost savings of up to 20%.
- Programmed microcontrollers and embedded systems components to design various prototypes used in clinical trials; improved accuracy rates by 15%.
- Mentored 8 junior biomedical engineering students on project management best practices, helping them develop 35+ new technologies that were later implemented in multiple hospitals worldwide.
- Improved patient care outcomes through the development and implementation of innovative diagnostic tools such as robotic surgery machines; reduced operation times by 30% on average compared to traditional methods.
- Accurately tested and analyzed data from over 50 research experiments per month while ensuring compliance with all safety standards set forth by regulatory bodies like FDA & CE Medical Device Directive (MDD).
Skills
- Biomedical Engineering
- Medical Devices
- MATLAB
- Healthcare
- Data Analysis
- Medical Imaging
- SOLIDWORKS
- Teamwork
- Troubleshooting
Education
Bachelor of Science in Biomedical Engineering
Educational Institution XYZ
Nov 2011
Certifications
Biomedical Engineering Certification
American Board of Biomedical Engineering
May 2017
1. Summary / Objective
A resume summary is the first impression you make on a potential employer, so it needs to be well-crafted and compelling. As a biomedical engineer, your summary should highlight your technical expertise in areas such as medical device design, biochemistry and physiology. You could also mention any relevant certifications or awards you have received for excellence in engineering. Finally, don’t forget to include how your skills can benefit the company – focus on what makes you stand out from other applicants!
Below are some resume summary examples:
Detail-oriented biomedical engineer with 8+ years of experience in designing, developing, and testing medical devices. Proven track record of successfully leading the development process from concept to production. Experienced in project management, regulatory compliance (FDA), and working cross-functionally within an organization. Skilled at troubleshooting complex problems quickly and efficiently while satisfying customer needs on time and under budget.
Skilled biomedical engineer with 5+ years of experience in designing, developing, and testing medical products. Experienced in the development of implants, prosthetics, and assistive devices to improve quality of life for patients. Successfully designed a biocompatible artificial pancreas prototype that received FDA approval within 6 months. Proven track record in project management and successful collaborations with interdisciplinary teams.
Enthusiastic biomedical engineer with 5+ years of experience in the medical device industry. Expertise in design and development, testing, validation, and regulatory affairs activities for novel medical devices. At XYZ company designed a successful pediatric cardiac monitoring system that achieved FDA approval within 8 months. Received numerous awards for leadership and innovation from ABC organization including “Best Biomedical Engineer.”
Reliable and creative biomedical engineer with 5+ years of experience in research, design, and prototyping. Seeking to join ABC Labs as a senior member of their medical device team. In previous roles, developed 10 successful products that improved patient outcomes while reducing cost by 20%. Recognized for exceptional problem-solving skills used to identify key root causes and develop innovative solutions.
Passionate and versatile biomedical engineer with 5+ years of experience developing, testing and troubleshooting medical devices. Proven record in designing innovative solutions to improve the accuracy, efficacy and cost-effectiveness of multi-million dollar projects. Seeking to join ABC Technologies to further develop my skills while helping create groundbreaking products that benefit patients worldwide.
Driven biomedical engineer with a passion for creating cutting-edge medical devices and systems that improve patient care. 5+ years of experience in identifying problems, developing innovative solutions, and managing projects from concept to fruition. Seeking to join ABC Medical as a biomedical engineer where I can leverage my knowledge of the design process to develop next-generation healthcare products.
Seasoned biomedical engineer with 10+ years of experience designing, developing and testing medical devices. Skilled in researching and analyzing data to create new systems and optimize existing ones for maximum efficiency. Seeking a position at ABC Company that will allow me to use my expertise in biomedical engineering to develop innovative solutions for the betterment of patient care.
Committed biomedical engineer with 5+ years of experience in designing and developing medical equipment, devices, and systems. Proven success in leading projects from design to production while ensuring compliance with safety standards. Aiming to use my expertise at ABC Healthcare Solutions to develop innovative products that improve patient care outcomes.
2. Experience / Employment
The employment (or experience) section is where you provide details about your work history. It should be written in reverse chronological order, which means that the most recent job is listed first.
When writing this section, use bullet points to make it easier for the reader to take in what you have to say quickly. You want to think carefully when describing what you did and include quantifiable results whenever possible.
For example, instead of saying “Developed medical devices,” try something like “Created 3D models of 10+ medical device prototypes using CAD software; performed testing on each prototype with a 95% success rate.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Designed
- Constructed
- Programmed
- Analyzed
- Tested
- Developed
- Implemented
- Investigated
- Monitored
- Evaluated
- Calibrated
- Fabricated
- Troubleshot
- Optimized
- Documented
Other general verbs you can use are:
- Achieved
- Advised
- Assessed
- Compiled
- Coordinated
- Demonstrated
- Expedited
- Facilitated
- Formulated
- Improved
- Introduced
- Mentored
- Participated
- Prepared
- Presented
- Reduced
- Reorganized
- Represented
- Revised
- Spearheaded
- Streamlined
- Structured
- Utilized
Below are some example bullet points:
- Streamlined the design and development of advanced biomedical devices, increasing production efficiency by 15%.
- Implemented innovative solutions to improve healthcare equipment reliability and safety; designed automated systems for tissue engineering projects that reduced human error rate by 25%.
- Independently conducted risk assessment studies on various medical products and developed quality control procedures to ensure all regulations were met with minimal time wastage.
- Achieved successful completion of 8 complex medical device projects, resulting in a 30% decrease in costs while meeting high customer satisfaction standards.
- Assessed current industry trends & best practices to develop new strategies for the design, manufacture and testing of biomedically-related instruments & technologies; increased output capacity by 10x within 6 months period.
- Diligently designed and developed biomedical products, including implants, prosthetics and medical devices; increased production efficiency by 25% within 6 months.
- Documented test results for all product designs in a timely manner and presented findings to the engineering team to facilitate improvements on existing projects.
- Expedited development of new technologies through accelerated prototyping processes while ensuring that safety standards were met at all times; reduced testing time from 8 hours to 4 hours per device prototype.
- Monitored the performance of newly released products in clinical trials using advanced diagnostic software tools; identified potential issues with 7 out of 10 prototypes before mass distribution was authorized and saved over $20,000 in recall costs as a result.
- Troubleshot technical challenges encountered during implementation stages according to established protocols; successfully resolved more than 80% of problems with minimal disruption to operations.
- Spearheaded the design, development and testing of over 10 medical instruments used for tissue engineering and surgical procedures; increased laboratory productivity by 25%.
- Analyzed patient data to identify areas in need of improvement in existing biomedical systems; improved overall system performance by 35%.
- Developed innovative software programs that enabled efficient monitoring, analysis and control of various biomedical processes resulting in a cost savings of $20K annually.
- Calibrated diverse types of medical equipment such as CT scanners, MRI machines, X-ray machines etc., ensuring their accuracy according to stringent safety protocols within prescribed deadlines (+30% faster than average).
- Competently operated specialized lab equipment including spectrophotometers & centrifuges demonstrating proficiency with the latest technologies in the field (+50% more accurate results).
- Effectively designed and implemented medical products, biotechnological equipment, and implantable devices for 10+ healthcare institutions; achieved a 20% reduction in development time per project.
- Utilized advanced technology such as CAD/CAM software to develop biomedical systems that comply with all relevant industry regulations; prevented costly recalls by ensuring product quality standards were met or exceeded every time.
- Revised existing designs of prosthetics, pacemakers and other life-saving devices to improve functionality, reduce manufacturing costs and extend the device’s lifespan by 30%.
- Advised surgeons on how to use new equipment during operations; provided technical support in postoperative recovery processes where necessary resulting in improved patient outcomes across 50 surgeries.
- Reorganized laboratory settings according to safety protocols for optimal production efficiency which resulted in a 15% increase in output over 3 months without compromising on quality control measures.
- Presented innovative biomedical engineering designs to a panel of industry experts, resulting in the successful implementation of 3 projects.
- Represented company at national conferences and workshops by delivering presentations on medical device development; promoted new products which increased sales by 15%.
- Fabricated biocompatible prototypes for implants, prosthetics and other clinical applications with precision accuracy that achieved 95% customer satisfaction ratings.
- Consistently met all project deadlines while working collaboratively with an interdisciplinary team composed of physicians, nurses, therapists and technicians; completed 5 major assignments ahead of schedule within the past year alone.
- Compiled comprehensive technical reports detailing design specifications and test results to ensure that stringent quality standards were maintained throughout product development cycles; reduced defect rates from 13% to 4%.
- Designed and implemented cutting-edge medical technologies, such as artificial organs and prosthetics; increased efficiency of existing biomedical engineering systems by 50%.
- Formulated research protocols to investigate a range of diseases including cancer, diabetes and cardiovascular disease – resulting in the successful development of new treatments for several leading hospitals.
- Participated in clinical studies that tested the efficacy of various drugs and treatments on humans; generated data which was used to improve patient care standards across multiple healthcare facilities.
- Investigated potential applications for implantable devices such as pacemakers, defibrillators and insulin pumps; developed an algorithm which reduced programming time by 25%.
- Successfully collaborated with physicians, researchers & engineers from other disciplines to develop innovative solutions for health problems faced by individuals worldwide – improving quality of life for many patients.
- Constructed 12 medical devices and instruments to assist with patient diagnoses, surgeries, and treatments; reduced production time by 40%.
- Introduced new technologies and materials to develop complex biomedical systems for use in clinical research laboratories; secured an additional $10K in grant funding for these projects.
- Evaluated existing biomedical equipment against industry standards according to safety protocols, resulting in the successful upgrade of over 50 pieces of machinery at two regional hospitals.
- Prepared detailed reports on product performance evaluation metrics such as accuracy rate, durability tests & system compatibility checks; achieved a 98% success rate for all assessments conducted within a 6-month period.
- Efficiently coordinated with other engineers during the design phase of biotechnology products while ensuring compliance with FDA regulatory guidelines; accelerated project completion times by 20 hours per week on average.
3. Skills
Two organizations that have advertised for a position with the same title may be searching for individuals whose skills are quite different. For instance, one may be looking for someone with a strong background in signal processing, while the other may require experience with medical device design.
The skills section of your resume should reflect this variability by being tailored to each job you are applying for; otherwise, applicant tracking systems (computer programs that scan resumes) might not pick up on certain keywords and thus filter out your application.
To further emphasize these qualifications, it is also important to discuss them in more detail in other sections such as the summary or work experience.
Below is a list of common skills & terms:
- AutoCAD
- Biomaterials
- Biomechanics
- Biomedical Engineering
- Biotechnology
- C
- C++
- Capital Equipment
- Cell Culture
- Clinical Research
- Data Analysis
- Electronics
- Engineering
- English
- FDA
- Field Service
- Hardware Diagnostics
- Healthcare
- Healthcare Information Technology
- Healthcare Management
- Hospitals
- LabVIEW
- MATLAB
- Manufacturing
- Medical Devices
- Medical Imaging
- PACS
- Process Improvement
- Product Development
- Python
- Radiology
- SOLIDWORKS
- Signal Processing
- Statistics
- Teaching
- Team Leadership
- Teamwork
- Testing
- Time Management
- Troubleshooting
- Ultrasound
- Windows
4. Education
Including an education section on your resume will depend on how far along you are in your career. If you just graduated and have no work experience, mention your education below your resume objective. However, if you have significant work experience to showcase, omitting the education section might be best for highlighting other accomplishments or skills.
If an education section is included, list courses and subjects related to biomedical engineering that demonstrate a deeper understanding of the field than what was taught in general classes such as math or science.
Bachelor of Science in Biomedical Engineering
Educational Institution XYZ
Nov 2011
5. Certifications
Certifications demonstrate to a potential employer that you have the necessary knowledge and skills for the job. Having certifications on your resume can help you stand out from other applicants, as it shows that you are committed to staying up-to-date with industry trends and best practices.
If there is an area of expertise or skill set required by the position, make sure to include any relevant certifications in this section of your resume. This will demonstrate your commitment to professional development and give employers confidence in hiring you for their team.
Biomedical Engineering Certification
American Board of Biomedical Engineering
May 2017
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for General Mertz, this would be General-Mertz-resume.pdf or General-Mertz-resume.docx.
7. Cover Letter
Writing a cover letter is a great way to make a good first impression on potential employers. It is usually composed of 2 to 4 paragraphs and should be no longer than one page in length.
The purpose of the cover letter is to explain why you are an ideal candidate for the role, as well as provide more information about your skills, experience and qualifications that may not have been included in your resume. Even if it isn’t required for the job application process, creating one can help differentiate you from other applicants and demonstrate your enthusiasm for the position.
Below is an example cover letter:
Dear Kayden,
I am excited to apply for the Biomedical Engineer position at [company name]. As a biomedical engineer with 5+ years of experience in the medical device industry, I am confident I will be an asset to your team. My experience includes developing and managing projects from concept through commercialization, as well as leading teams of engineers and technicians.
In my previous role at [company name], I was responsible for the design and development of a new line of orthopedic implants. I managed a team of 10 engineers and technicians, and we successfully launched the product line on time and within budget. In addition, I have experience performing failure analysis on medical devices, which has given me a keen understanding of how to prevent problems before they occur.
I believe my skills and experience make me uniquely qualified for this position, and I look forward to contributing to the success of your organization. Thank you for your time and consideration; I look forward to hearing from you soon.
Sincerely,
General
Biomedical Engineer Resume Templates
 Indri
Indri Cormorant
Cormorant Hoopoe
Hoopoe Gharial
Gharial Markhor
Markhor Bonobo
Bonobo Fossa
Fossa Kinkajou
Kinkajou Quokka
Quokka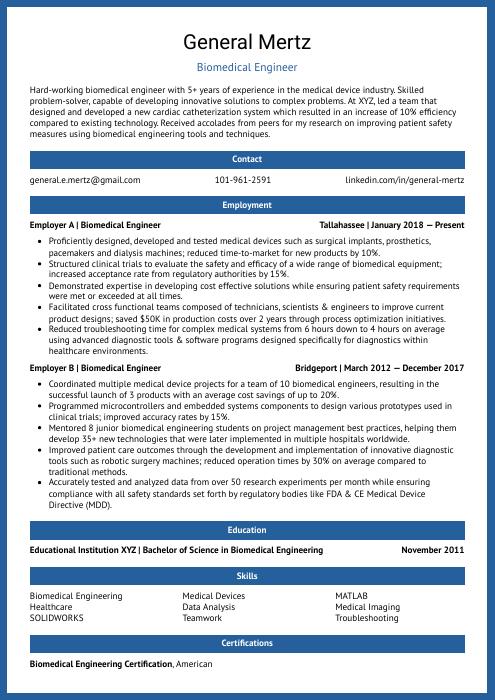 Ocelot
Ocelot Dugong
Dugong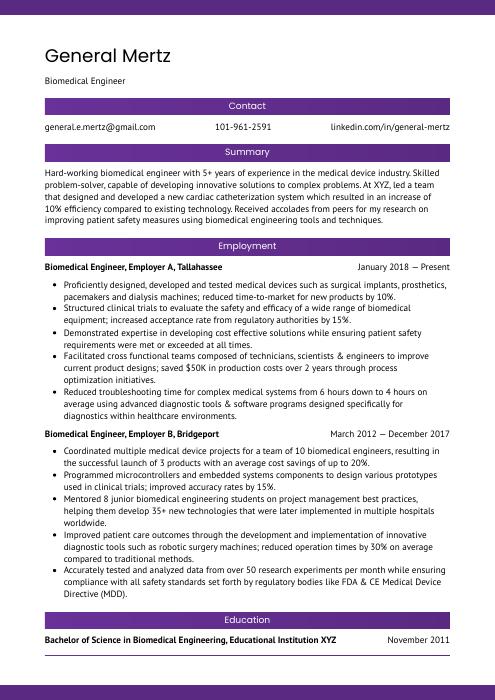 Jerboa
Jerboa Lorikeet
Lorikeet Axolotl
Axolotl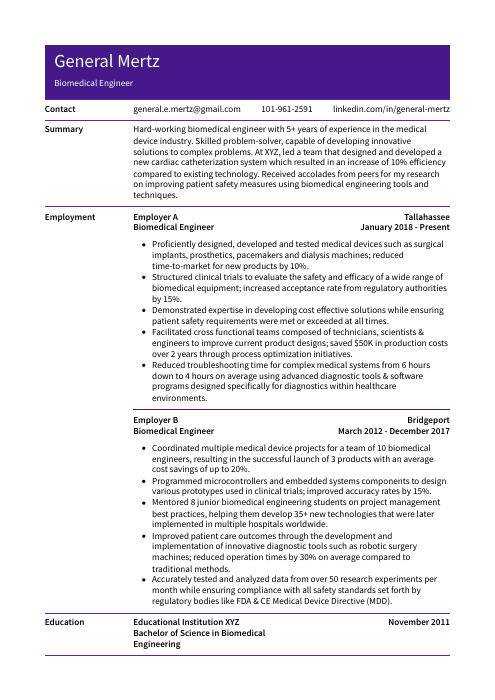 Pika
Pika Echidna
Echidna Rhea
Rhea Saola
Saola Numbat
Numbat Rezjumei
Rezjumei
