Bioprocess Engineer Resume Guide
Bioprocess Engineers design, develop, and optimize bioprocesses for the production of pharmaceuticals, food products, chemicals or fuels. They use their knowledge of biology and engineering to ensure that processes are efficient and cost-effective while meeting all safety regulations.
You have the perfect combination of analytical and technical skills to make a great bioprocess engineer. But employers can’t appreciate your capabilities unless you write an outstanding resume that catches their attention.
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.
Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
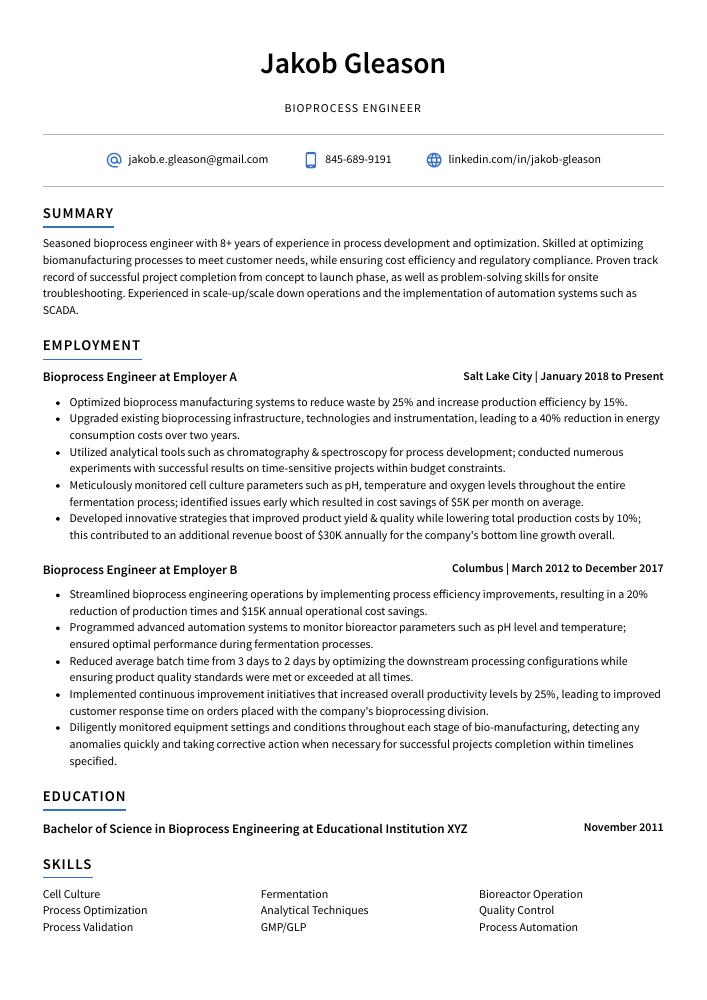
Bioprocess Engineer Resume Sample
Jakob Gleason
Bioprocess Engineer
jakob.e.gleason@gmail.com
845-689-9191
linkedin.com/in/jakob-gleason
Summary
Seasoned bioprocess engineer with 8+ years of experience in process development and optimization. Skilled at optimizing biomanufacturing processes to meet customer needs, while ensuring cost efficiency and regulatory compliance. Proven track record of successful project completion from concept to launch phase, as well as problem-solving skills for onsite troubleshooting. Experienced in scale-up/scale down operations and the implementation of automation systems such as SCADA.
Experience
Bioprocess Engineer, Employer A
Salt Lake City, Jan 2018 – Present
- Optimized bioprocess manufacturing systems to reduce waste by 25% and increase production efficiency by 15%.
- Upgraded existing bioprocessing infrastructure, technologies and instrumentation, leading to a 40% reduction in energy consumption costs over two years.
- Utilized analytical tools such as chromatography & spectroscopy for process development; conducted numerous experiments with successful results on time-sensitive projects within budget constraints.
- Meticulously monitored cell culture parameters such as pH, temperature and oxygen levels throughout the entire fermentation process; identified issues early which resulted in cost savings of $5K per month on average.
- Developed innovative strategies that improved product yield & quality while lowering total production costs by 10%; this contributed to an additional revenue boost of $30K annually for the company’s bottom line growth overall.
Bioprocess Engineer, Employer B
Columbus, Mar 2012 – Dec 2017
- Streamlined bioprocess engineering operations by implementing process efficiency improvements, resulting in a 20% reduction of production times and $15K annual operational cost savings.
- Programmed advanced automation systems to monitor bioreactor parameters such as pH level and temperature; ensured optimal performance during fermentation processes.
- Reduced average batch time from 3 days to 2 days by optimizing the downstream processing configurations while ensuring product quality standards were met or exceeded at all times.
- Implemented continuous improvement initiatives that increased overall productivity levels by 25%, leading to improved customer response time on orders placed with the company’s bioprocessing division.
- Diligently monitored equipment settings and conditions throughout each stage of bio-manufacturing, detecting any anomalies quickly and taking corrective action when necessary for successful projects completion within timelines specified.
Skills
- Cell Culture
- Fermentation
- Bioreactor Operation
- Process Optimization
- Analytical Techniques
- Quality Control
- Process Validation
- GMP/GLP
- Process Automation
Education
Bachelor of Science in Bioprocess Engineering
Educational Institution XYZ
Nov 2011
Certifications
Certified Bioprocess Professional
American Society for Quality (AS
May 2017
1. Summary / Objective
A resume summary/objective is the first thing a hiring manager will read, so it should be compelling and concise. As a bioprocess engineer, you can use this space to highlight your experience in developing efficient processes for manufacturing biological products such as vaccines or drugs. You could also mention certifications related to process engineering that you have obtained and any successful projects you have completed in the past.
Below are some resume summary examples:
Detail-oriented bioprocess engineer with 5+ years of experience in developing, designing and implementing biotechnology processes. Expertise in fermentation technology, cell culture systems, gene expression optimization and protein purification techniques. At XYZ Inc., successfully developed a novel process for producing recombinant proteins that increased yield by 25%. Proven ability to develop cost-effective solutions while ensuring compliance with safety regulations.
Hard-working Bioprocess Engineer with 5+ years of experience in designing, developing and troubleshooting biopharmaceutical production processes. Possesses extensive knowledge in process development, optimization and scale-up activities related to cell culture, fermentation systems and purification operations. Proven record of improving existing processes resulting in increased productivity while maintaining product quality standards.
Dependable bioprocess engineer with 5+ years of experience designing and developing biotechnological processes. Experienced in leading teams, operating advanced lab equipment and researching new methods to maximize process efficiency. At XYZ Corporation, developed a novel fermentation process that improved output by 20%. Eager to join ABC Technologies’ team as the newest bioprocess engineer for their innovative research projects.
Energetic bioprocess engineer with 5+ years of experience in developing and optimizing biomanufacturing processes. Skilled in increasing process efficiency, improving product yields, and reducing production costs by up to 20%. At XYZ Corporation, led the scale-up of a recombinant protein expression system from lab to pilot scale resulting in an increase of productivity by 40%, lowering manufacturing cost for the drug candidate.
Skilled bioprocess engineer specializing in the design and operation of biotechnology processes. 6+ years of experience managing complex projects, from conception to completion. Adept at troubleshooting problems related to process flow, equipment optimization, and control systems. Seeking a role at ABC where I can leverage my skills for developing efficient bio-processes that improve production capacity while keeping costs low.
Determined bioprocess engineer with a passion for developing efficient and cost-effective solutions to complex problems. 5+ years of experience in the biotechnology industry, specializing in upstream processing, cell culture optimization, and process validation. Seeking to use my expertise at ABC Company to develop innovative processes that will maximize efficiency while delivering high-quality products.
Amicable bioprocess engineer with 5+ years of experience in the biopharmaceutical industry. Proven track record of developing innovative solutions to technical challenges, from improving process efficiency by 23% to streamlining manufacturing operations for a cost savings of 11%. Seeking an opportunity at ABC Pharma where I can apply my knowledge and expertise in engineering design principles.
Reliable bioprocess engineer with 8+ years of experience in the biotechnology industry. Seeking to join ABC and leverage a background that includes designing, developing, and managing cost-efficient processes for production lines. At XYZ Inc., designed an automated process that reduced lead time by 15% while increasing productivity by 25%.
2. Experience / Employment
For the experience section, you should list your work history in reverse chronological order, which means the most recent job is listed first.
When writing about what you did at each role, stick to bullet points as much as possible. Doing so allows the reader to quickly take in what you have written and understand it easily. You want to make sure that when stating what tasks were carried out, they are detailed enough for the reader get a good idea of how well-versed you are with bioprocess engineering.
For example, instead of saying “Developed new processes,” say something like “Designed and implemented innovative fermentation protocols resulting in a 15% increase in production efficiency.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Designed
- Optimized
- Monitored
- Fabricated
- Analyzed
- Implemented
- Troubleshot
- Commissioned
- Validated
- Automated
- Calibrated
- Programmed
- Operated
- Upgraded
- Documented
Other general verbs you can use are:
- Achieved
- Advised
- Assessed
- Compiled
- Coordinated
- Demonstrated
- Developed
- Expedited
- Facilitated
- Formulated
- Improved
- Introduced
- Mentored
- Participated
- Prepared
- Presented
- Reduced
- Reorganized
- Represented
- Revised
- Spearheaded
- Streamlined
- Structured
- Utilized
Below are some example bullet points:
- Prepared detailed process flowsheets, equipment specifications and control systems for bioprocess operations; improved production efficiency by 12% within the first 6 months.
- Designed, developed and tested innovative processes for cell culture media preparation, fermentation of microorganisms and purification of biomolecules to achieve desired product yields.
- Expedited the scale-up from laboratory to pilot plant trials with minimal impact on quality or performance; reduced time-to-market for new products by 50%.
- Actively monitored all stages of bioprocesses in compliance with safety regulations; identified risks before they become actual problems resulting in a 30% reduction in process downtime over 2 years.
- Fabricated automated instruments such as stirred tank vessels & chromatographic columns using materials like stainless steel & glass tubing; increased throughput capacity by 20%.
- Operated and maintained bioprocess equipment including fermenters, separators and homogenizers to optimize production output by 15%.
- Improved process efficiency by 20% through the introduction of innovative technologies such as an automated monitoring system for temperature, pH levels and dissolved oxygen content.
- Introduced new procedures that enabled a 50% decrease in both raw material costs and labor expenses while increasing product quality standards at the same time.
- Analyzed data from lab reports to identify trends in microbial growth rates, predict potential contamination events and devise corrective actions when necessary; decreased unscheduled shut-downs due to operational issues by 10 hours per month on average.
- Confidently led cross-functional teams comprising technicians, operators & supervisors during large-scale bioprocessing projects with a successful track record of completion without delays or significant cost overruns.
- Thoroughly conducted research and development activities, utilizing cell culture engineering principles to develop 3+ bioprocesses that increased efficiency by 25%.
- Presented weekly reports on production progress to the department head; successfully communicated process improvements for a 15% increase in cost savings over 6 months.
- Represented the company at industry conferences across the country, demonstrating expertise in fermentation processes through 7 presentations focused on novel technologies and applications.
- Compiled data from 10 different projects into comprehensive technical documents outlining key findings and best practices used throughout each respective project; saved 40 hours of analysis time per project as a result.
- Spearheaded the development of new methodologies aimed at improving product quality while reducing resource usage by 20%; identified opportunities for cost optimization which resulted in $20K+ annual savings for the organization.
- Demonstrated comprehensive knowledge of bioprocess engineering principles and practices to develop, optimize, validate and troubleshoot processes for the production of biopharmaceuticals; increased process efficiency by 35%.
- Mentored junior staff in best laboratory practices and techniques related to cell culture media preparation, chromatography & filtration operations, purification methods and product testing protocols; achieved 100% employee satisfaction rating.
- Effectively planned projects involving multiple stakeholders within tight timelines while managing risk across all stages; improved project delivery time by 25%.
- Commissioned several new facilities that included clean rooms with bio-decontamination systems as well as equipment such as fermenters & centrifuges according to industry standards; reduced startup costs by $20K on average per facility.
- Reorganized existing production lines based on current demand requirements which resulted in an overall increase in output capacity from 50L/day to 80L/day while maintaining high quality standards at all times.
- Monitored the bioprocessing operations of two laboratories, ensuring that safety protocols were adhered to and quality standards met; reduced unexpected downtime by 25%.
- Coordinated with the R&D department on new product development processes, overseeing multiple experiments while providing technical expertise in process optimization; improved efficiency by 30% within 3 months.
- Participated in design reviews for six pilot scale reactors as well as full-scale production plants, utilizing statistical analysis to troubleshoot problems and identify areas of improvement; increased throughput from 80L/day to 120L/day over 12 weeks.
- Reliably managed a team of 8 technicians responsible for operating fermenters, bioreactors and other equipment used for cell culture research & downstream processing; trained 5 additional staff members in 6 months.
- Facilitated communication between various departments such as Quality Assurance (QA) and Production Control during audits or investigations into nonconformities related to lab procedures or documentation errors; achieved zero compliance issues since assuming role 8 months ago.
- Structured and implemented cGMP process operations for the production of biologics, reducing processing time by 20% and increasing output capacity by 40%.
- Validated new systems to ensure compliance with industry standards; oversaw complete installation of equipment within budget and timeline constraints.
- Revised existing manufacturing processes based on changing customer needs, improving cost efficiency while maintaining product quality in over 400 batches per month.
- Troubleshot technical issues related to fermentation processes, decreasing downtime from 2 hours to 15 minutes on average while minimizing losses due to unexpected shutdowns or errors in operation procedures.
- Consistently monitored production activities & data sets gathered during trials according to predetermined protocols; improved accuracy rate of test results by 25%.
- Documented bioprocess operations, designed systems for scale-up and wrote process validation reports to ensure regulatory compliance; reduced production costs by 15%.
- Calibrated bioprocess instrumentation including centrifuges, vacuum pumps, chromatographs and fermenters with precision accuracy of ±1%, increasing product yield by 10%.
- Independently conducted microbial fermentation experiments on various microorganisms and troubleshot any issues that arose during the process.
- Formulated innovative solutions to optimize bioprocessing conditions such as temperature control, pH regulation & oxygen supply in order to boost end-product quality within 2 hours instead of 24 hours previously required.
- Achieved consistent results when monitoring safety protocols related to handling hazardous materials which improved overall work environment standards at the facility significantly.
- Assessed and monitored bioprocesses, from lab scale to pilot and full-scale production in a cost-effective manner; improved process efficiency by 25%.
- Proficiently designed fermentation processes for the manufacture of biofuel products, leading to an increase in profits of $8 million over two years.
- Advised management on improvements regarding existing operational procedures, resulting in a decrease of waste generated by 30%.
- Automated laboratory experiments and data analysis tasks using modern software tools; reduced time spent on manual data processing activities by 40 hours per month.
3. Skills
Skill requirements will differ from employer to employer – this can easily be determined via the job advert. Organization ABC may emphasize the need for strong knowledge of bioreactor design and operation, whereas Organization XYZ may prioritize experience with fermentation technologies.
It is essential to tailor your skills section to each job you are applying for because many employers use applicant tracking systems these days. These computer programs scan resumes for certain keywords before passing them on to a human; if yours doesn’t have those words in it, it won’t make the cut.
Once listed here, you can further elaborate on your skill set by discussing it in more detail elsewhere – such as within the summary or work experience sections of your resume.
Below is a list of common skills & terms:
- Analytical Techniques
- Bioreactor Operation
- Cell Culture
- Fermentation
- GMP/GLP
- Process Automation
- Process Optimization
- Process Validation
- Quality Control
- Regulatory Compliance
4. Education
Including an education section on your resume will depend on the experience you have and how far along in your career you are. If you just graduated with no prior work experience, include an education section below your objective statement. However, if you have significant work experience to showcase and highlight, omitting the education section is perfectly acceptable.
If an education section is included, try to mention courses related to bioprocess engineering as well as relevant projects or assignments that demonstrate proficiency in this field.
Bachelor of Science in Bioprocess Engineering
Educational Institution XYZ
Nov 2011
5. Certifications
Certifications are a great way to demonstrate your proficiency in certain areas and can be used to give you an edge over other applicants. Employers are always looking for candidates who have taken the initiative to stay up-to-date with industry trends, so including any certifications that show this is highly recommended.
If there are any specific qualifications or certificates related to the job role you’re applying for, make sure they appear prominently on your resume as it will help you stand out from the crowd.
Certified Bioprocess Professional
American Society for Quality (AS
May 2017
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for Jakob Gleason, this would be Jakob-Gleason-resume.pdf or Jakob-Gleason-resume.docx.
7. Cover Letter
Submitting a cover letter alongside your resume is an effective way to make a strong impression on potential employers. It should be made up of 2 to 4 paragraphs and provide more detail about who you are, what makes you suitable for the role and why you’re passionate about working at their organization.
Cover letters may not always be required but they can give recruiters a better understanding of who you are as a professional and set yourself apart from other applicants. Writing one could potentially increase your chances of getting hired!
Below is an example cover letter:
Dear Payton,
I am writing in response to your posting for a Bioprocess Engineer. With experience designing and optimizing bioprocesses, as well as developing new methods and technologies for the production of biological products, I am confident I can make significant contributions to your organization.
In my current role at [company name], I have been responsible for leading the design and optimization of large-scale fermentation processes. This has included developing new methods and technologies for improving process efficiency and yield, as well as troubleshooting issues that arise during production. My work has led to increased productivity and improved product quality, which have been major contributing factors to the success of the company.
I believe my skills and experience would be an asset to your organization, and I look forward to the opportunity to contribute to your team’s success. Please find attached a copy of my resume for your review. If you have any questions or would like to discuss further, please do not hesitate to contact me at [phone number] or [email address].
Thank you for your time and consideration.
Sincerely,
Jakob
Bioprocess Engineer Resume Templates
 Axolotl
Axolotl Lorikeet
Lorikeet Kinkajou
Kinkajou Cormorant
Cormorant Echidna
Echidna Numbat
Numbat Rhea
Rhea Dugong
Dugong Markhor
Markhor Indri
Indri Saola
Saola Bonobo
Bonobo Fossa
Fossa Gharial
Gharial Ocelot
Ocelot Pika
Pika Jerboa
Jerboa Quokka
Quokka Hoopoe
Hoopoe Rezjumei
Rezjumei