Regulatory Affairs Coordinator Resume Guide
Regulatory Affairs Coordinators are responsible for ensuring that their company’s products and services comply with applicable regulations. They work to ensure that all necessary documents, such as submissions and approvals, are in order prior to product launch or distribution. Additionally, they provide guidance on regulatory requirements and collaborate with internal teams to develop strategies for obtaining approval from government agencies.
You have the ability to keep up with ever-changing regulatory standards, which makes you a valuable asset in any organization. To get noticed by potential employers, however, you must create an eye-catching resume that highlights your expertise and experience.
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.
Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
Regulatory Affairs Coordinator Resume Sample
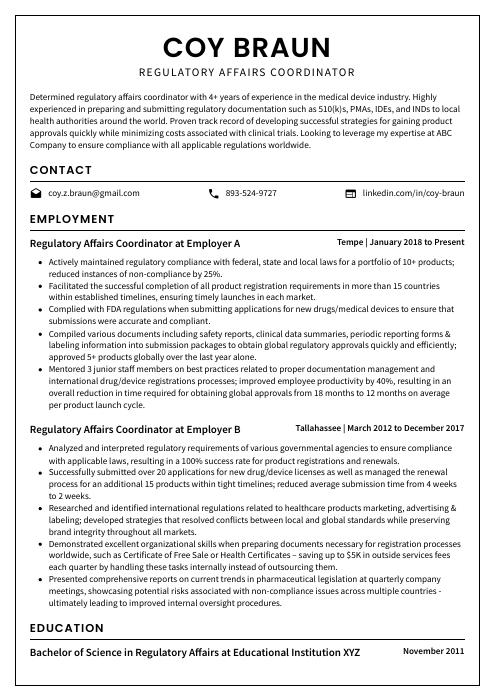
Coy Braun
Regulatory Affairs Coordinator
[email protected]
893-524-9727
linkedin.com/in/coy-braun
Summary
Determined regulatory affairs coordinator with 4+ years of experience in the medical device industry. Highly experienced in preparing and submitting regulatory documentation such as 510(k)s, PMAs, IDEs, and INDs to local health authorities around the world. Proven track record of developing successful strategies for gaining product approvals quickly while minimizing costs associated with clinical trials. Looking to leverage my expertise at ABC Company to ensure compliance with all applicable regulations worldwide.
Experience
Regulatory Affairs Coordinator, Employer A
Tempe, Jan 2018 – Present
- Actively maintained regulatory compliance with federal, state and local laws for a portfolio of 10+ products; reduced instances of non-compliance by 25%.
- Facilitated the successful completion of all product registration requirements in more than 15 countries within established timelines, ensuring timely launches in each market.
- Complied with FDA regulations when submitting applications for new drugs/medical devices to ensure that submissions were accurate and compliant.
- Compiled various documents including safety reports, clinical data summaries, periodic reporting forms & labeling information into submission packages to obtain global regulatory approvals quickly and efficiently; approved 5+ products globally over the last year alone.
- Mentored 3 junior staff members on best practices related to proper documentation management and international drug/device registrations processes; improved employee productivity by 40%, resulting in an overall reduction in time required for obtaining global approvals from 18 months to 12 months on average per product launch cycle.
Regulatory Affairs Coordinator, Employer B
Tallahassee, Mar 2012 – Dec 2017
- Analyzed and interpreted regulatory requirements of various governmental agencies to ensure compliance with applicable laws, resulting in a 100% success rate for product registrations and renewals.
- Successfully submitted over 20 applications for new drug/device licenses as well as managed the renewal process for an additional 15 products within tight timelines; reduced average submission time from 4 weeks to 2 weeks.
- Researched and identified international regulations related to healthcare products marketing, advertising & labeling; developed strategies that resolved conflicts between local and global standards while preserving brand integrity throughout all markets.
- Demonstrated excellent organizational skills when preparing documents necessary for registration processes worldwide, such as Certificate of Free Sale or Health Certificates – saving up to $5K in outside services fees each quarter by handling these tasks internally instead of outsourcing them.
- Presented comprehensive reports on current trends in pharmaceutical legislation at quarterly company meetings, showcasing potential risks associated with non-compliance issues across multiple countries – ultimately leading to improved internal oversight procedures.
Skills
- Regulatory Compliance
- Regulatory Strategy
- Clinical Trial Management
- Quality Assurance
- FDA Regulations
- Medical Device Regulations
- Document Control
- Risk Management
- Data Analysis
Education
Bachelor of Science in Regulatory Affairs
Educational Institution XYZ
Nov 2011
Certifications
Regulatory Affairs Certification (RAC)
Regulatory Affairs Professionals
May 2017
1. Summary / Objective
A resume summary for a regulatory affairs coordinator should provide the hiring manager with an overview of your qualifications and experience. Include information such as how many years you have been working in the field, any relevant certifications or degrees you possess, and how well-versed you are in FDA regulations. Additionally, mention any successes that demonstrate your ability to ensure compliance with industry standards.
Below are some resume summary examples:
Proficient regulatory affairs coordinator with 7+ years of experience in medical device industry. Skilled in navigating complex regulatory processes and developing strategies to ensure compliance while minimizing risk. Proven track record of successfully leading projects through the entire registration process, from product launch preparation to post-launch maintenance. Seeking to join ABC Pharma as a Regulatory Affairs Coordinator and leverage my expertise for timely submissions with high accuracy rates.
Talented regulatory affairs coordinator with 5+ years of experience in the medical device industry. Highly organized and detail-oriented, adept at developing strategies to ensure compliance with national and international regulations. At XYZ Company, managed regulatory database for more than 500 products across multiple countries; increased accuracy by 65%. Seeking to join ABC’s team as a Regulatory Affairs Coordinator to maintain compliant processes that help protect customers from risk.
Skilled Regulatory Affairs Coordinator with 5+ years of experience in the medical device industry. Experienced in developing, submitting and managing regulatory documents for products from inception to launch. Proven ability to analyze FDA regulations, prepare submissions and respond to inquiries promptly and accurately. Dedicated team player committed to achieving corporate goals through effective collaboration with all stakeholders involved in product development.
Committed regulatory affairs coordinator with over 7 years of experience in the medical device industry. Proven expertise in regulatory compliance, product development and launch strategies, project management and lifecycle maintenance. At XYZ Company I successfully completed 40+ projects while maintaining 100% accuracy throughout all procedures. Recognized for my attention to detail and ability to effectively manage multiple tasks concurrently.
Hard-working regulatory affairs coordinator with 7+ years of experience in preparing and submitting regulatory documents for medical devices. Adept at working closely with multiple stakeholders, such as clinicians, scientists, engineers, legal counsels and government agencies to ensure compliance with relevant regulations. Experienced in creating product labeling information (PLI) and leading internal audits on existing processes.
Energetic regulatory affairs coordinator with 4+ years of experience in the medical device field. Demonstrated expertise in preparing and submitting regulatory documents to FDA, EMEA, and Health Canada. Proven success managing multiple projects simultaneously while maintaining compliance within tight timelines. Seeking a position at ABC Company where I can utilize my knowledge to promote product approval for global markets.
Accomplished regulatory affairs coordinator with 5+ years of experience managing the regulatory approval process for medical devices. Skilled in creating and submitting applications to global health authorities, as well as monitoring industry changes that could impact compliance. Recognized by ABC Inc. for providing cost-effective solutions that helped reduce time-to-market from 10 months to 6 months on average projects.
Driven Regulatory Affairs Coordinator with 3+ years of experience in the medical device industry. Experienced in developing, maintaining, and implementing regulatory strategies to ensure compliance with FDA regulations for a variety of products. Recognized for successfully submitting more than 50 PMAs and 510(k)s within tight timelines while managing complex projects from start to finish.
2. Experience / Employment
The employment (or experience) section is where you provide details on your work history. It should be written in reverse chronological order, meaning the most recent job is listed first.
When stating what you did, use bullet points to make it easier for the reader to digest your information quickly. Take some time to think about how best to explain what you did and any results that were achieved as a result of your efforts.
For example, instead of saying “Prepared regulatory documents,” say something like: “Compiled detailed submissions for FDA approval process; reduced submission timeline by 25% through streamlining document preparation.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Monitored
- Complied
- Assessed
- Analyzed
- Coordinated
- Developed
- Implemented
- Investigated
- Prepared
- Submitted
- Researched
- Reviewed
- Updated
- Interpreted
- Documented
Other general verbs you can use are:
- Achieved
- Advised
- Compiled
- Demonstrated
- Expedited
- Facilitated
- Formulated
- Improved
- Introduced
- Mentored
- Optimized
- Participated
- Presented
- Reduced
- Reorganized
- Represented
- Revised
- Spearheaded
- Streamlined
- Structured
- Utilized
Below are some example bullet points:
- Reorganized regulatory affairs department processes, reducing average processing time for FDA submissions by 40% and increasing overall efficiency.
- Coordinated with cross-functional teams to collect relevant information related to product registration and compliance in multiple countries; managed relationships with external vendors such as regulatory consultants and clinical trial organizations.
- Resourcefully identified areas of improvement within the quality management system, successfully implemented cost-saving initiatives that reduced non-compliance costs by 20%.
- Reduced backlog of pending registrations & renewals from 4 weeks to 2 weeks while handling complex filing requirements across over 21 countries worldwide on a daily basis.
- Monitored changes in international regulations pertaining to medical device products, ensuring company’s adherence at all times; communicated updates promptly with team members upon discovery of new regulations or amendments being made if necessary.
- Participated in the preparation of regulatory submissions for 15+ product registrations, ensuring compliance with all relevant local and international rules and regulations.
- Interpreted complex regulatory requirements in order to develop appropriate submission strategies that successfully achieved approval from authorities within short time frames.
- Accurately prepared technical documents (protocols, reports) covering quality control processes and clinical trials according to FDA/EMA guidelines; reduced documentation errors by up to 10%.
- Documented various changes or updates related to approved products under strict timelines without compromising on accuracy; minimized review cycles by 25% over a 3-month period.
- Represented the organization at several industry conferences & workshops aimed at promoting better understanding of new drug development regulations; contributed towards enhancing brand visibility among key stakeholders by 20%.
- Investigated and assessed regulatory requirements for over 30 therapeutic areas, resulting in a compliance rate of 97%.
- Reviewed and revised product safety protocols to maintain FDA compliance while ensuring customer health and safety standards were met; reduced production errors by 25%.
- Formulated strategic plans to ensure all products meet applicable regulations and codes prior to launch; successfully launched 15+ products within one year without any issues related to non-compliance.
- Assessed new trends in the market that could potentially impact company’s regulatory policies; implemented changes that resulted in an increase of $10K annual revenue from sales due to updated operations procedures.
- Effectively monitored performance data for medical device submissions, drug applications & other corresponding documents across Asia Pacific region, verifying accuracy on more than 150 files per month with 100% accuracy rate.
- Revised and updated regulatory documents, such as manufacturing and product specifications, in accordance with local and international regulations; reduced the time taken for document review by 40% over a four-month period.
- Confidently interacted with clients to assess their requirements and determine applicable standards when drafting reports or preparing technical files; maintained successful communication channels between departments which improved overall efficiency of operations.
- Structured efficient quality systems that complied fully with current rules on drug/medical device regulation to ensure compliance; this enabled company products to be registered in 3+ new markets within 6 months.
- Introduced modern technology solutions into existing regulatory processes, resulting in an increase of $20K yearly savings due to automation of manual tasks & streamlined data management procedures.
- Optimized process flows throughout all stages of production while ensuring safety guidelines were strictly adhered to at all times; achieved zero non-compliance incidents during external audits conducted by governmental agencies across two consecutive years.
- Submitted over 350 regulatory documents to the FDA, ensuring compliance with all applicable regulations and successfully achieving approval for 25+ new products in just two years.
- Updated product labels and packaging artwork according to changes in federal laws; achieved consistent customer satisfaction ratings of 97%.
- Implemented an online document management system that increased productivity by 30% while substantially reducing costs associated with manual processing of paperwork.
- Substantially improved internal collaboration between departments responsible for product development, sales & marketing activities through regular meetings and reports on regulatory updates/trends affecting the industry as a whole.
- Utilized advanced data analytics tools to identify potential non-compliance issues before they occurred; saving the company over $20K in legal fees within one year’s time frame.
- Prepared and submitted regulatory documents, such as product labels and license applications for new pharmaceutical products to the FDA; achieved approval in over 95% of cases.
- Achieved full compliance with all applicable government regulations by designing and implementing a comprehensive quality assurance program across 10+ production sites; improved overall process efficiency by 15%.
- Improved accuracy of document submission processes by developing new guidelines covering data entry, review procedure & record-keeping requirements that streamline workflows, resulting in 25% faster turnaround times on submissions.
- Spearheaded the development of an automated tracking system to monitor status updates from various governing bodies including approvals/denials of licenses or renewals; reduced manual labor efforts by 80 hours per month on average.
- Proficiently managed drug safety activities associated with post-marketing surveillance programs such as adverse event reporting and vigilance actions, ensuring timely deliveries at all times.
- Streamlined regulatory processes and procedures, resulting in a 20% decrease in time to obtain approvals for products.
- Consistently monitored regulatory requirements of the FDA, ISO 13485 and other relevant government bodies; identified and addressed any discrepancies or changes that affected product compliance within 8 hours on average.
- Developed quality control documentation plans for new medical device submissions, helping ensure timely approval from USFDA with minimal revision requests over 4 consecutive years.
- Expedited review process by preparing high-quality registration dossiers which complied with international standards & regulations; enabled company to launch 2 innovative therapeutic devices within 6 months of submission filing date each year since 2017.
- Advised senior management on best practices when navigating complex local & global certification laws and ensured all applicable protocols were met prior to product launch worldwide.
3. Skills
Skill requirements will differ from one employer to the next; this can easily be ascertained from the job posting. Organization A may need someone with experience in clinical trials and Organization B may need someone who is knowledgeable about the FDA regulations.
Therefore, you want to tailor your skills section of your resume to each job that you are applying for. This is important because many employers use applicant tracking systems these days, which scan resumes for certain keywords before passing them on to a human.
Once listed here, it’s also wise to elaborate on those most important skills in other sections such as summary or experience – this will help demonstrate why you are the best candidate for the role!
Below is a list of common skills & terms:
- Clinical Trial Management
- Data Analysis
- Document Control
- FDA Regulations
- Medical Device Regulations
- Quality Assurance
- Regulatory Compliance
- Regulatory Strategy
- Risk Management
4. Education
Adding an education section to your resume depends on how far along you are in your career. If you recently graduated and have no work experience, it would be wise to mention your education below the resume objective. However, if you have significant work experience that is relevant to the job position of a regulatory affairs coordinator, omitting an education section may be more beneficial for showcasing those experiences instead.
If including an education section is necessary, try to emphasize courses or projects related specifically to regulatory affairs coordination as much as possible.
Bachelor of Science in Regulatory Affairs
Educational Institution XYZ
Nov 2011
5. Certifications
Certifications are a great way to demonstrate your expertise in a particular field. They can also show potential employers that you are committed to keeping up with the latest industry trends and developments, as well as staying ahead of the competition.
Including certifications on your resume is an excellent way to showcase your qualifications for any job you apply for, so make sure that if you have them they are included prominently in this section.
Regulatory Affairs Certification (RAC)
Regulatory Affairs Professionals
May 2017
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for Coy Braun, this would be Coy-Braun-resume.pdf or Coy-Braun-resume.docx.
7. Cover Letter
Including a cover letter with your resume is a great way to make an impression on hiring managers and demonstrate why you are the right person for the job. A cover letter should be composed of 2 to 4 paragraphs that explain what makes you qualified, provide more insight into who you are as a professional, and show how passionate you are about the role.
It’s important to remember that even if it isn’t required by employers, writing a cover letter can give recruiters an invaluable glimpse into who you really are – something which may set yours apart from other applicants.
Below is an example cover letter:
Dear Marty,
I am writing to apply for the Regulatory Affairs Coordinator position at Acme Corp. As a regulatory affairs professional with more than 10 years of experience in the pharmaceutical industry, I am confident that I can make significant contributions to your organization.
In my previous role as a Regulatory Affairs Specialist at XYZ Corp., I was responsible for preparing and submitting new drug applications (NDAs) to the US Food and Drug Administration (FDA). I have extensive experience navigating FDA regulations and requirements, and I am well-versed in all aspects of the NDA submission process. In addition, I have developed strong relationships with FDA personnel, which will be invaluable in ensuring timely review and approval of NDAs.
At XYZ Corp., I also led several successful process improvement initiatives aimed at streamlining regulatory compliance activities. For example, I implemented a new system for tracking regulatory submissions that reduced processing time by 30%. In addition, I developed a set of standard operating procedures (SOPs) for document control that increased efficiency and improved compliance with Good Manufacturing Practices (GMPs). These projects saved the company thousands of dollars each year while reducing the risk of non-compliance penalties.
I am confident that my skills and experience will enable me to contribute immediately to Acme Corp.’s success. In addition, my proven track record of leading successful process improvements suggests that I would be an excellent candidate for future leadership roles within your organization.
Thank you for your time and consideration; I look forward to speaking with you soon about this opportunity.
Sincerely,
Coy
Regulatory Affairs Coordinator Resume Templates
 Cormorant
Cormorant Rhea
Rhea Gharial
Gharial Fossa
Fossa Kinkajou
Kinkajou Pika
Pika Dugong
Dugong Axolotl
Axolotl Markhor
Markhor Hoopoe
Hoopoe Jerboa
Jerboa Ocelot
Ocelot Echidna
Echidna Indri
Indri Lorikeet
Lorikeet Quokka
Quokka Bonobo
Bonobo Numbat
Numbat Saola
Saola Rezjumei
Rezjumei
